aCommercial preparations are described in IU, based on a potency assay in hypophysectomized rats in which depletion of adrenal ascorbic acid is measured after subcutaneous ACTH injection.
Although most efficacy studies of ACTH and steroids are retrospective, an expanding body of prospective data is available (12,18,27,55–65). Most published literature supports the hypothesis that the natural ACTH 1 to 39 peptide (p-ACTH) is superior to oral steroids. In randomized, controlled trials, spasms ceased in 42% to 87% of children treated with ACTH, compared with 29% to 33% of children treated with prednisone (49,50). In these studies, the relapse rates were 15% to 31% for ACTH and 29% to 33% for prednisone. The United Kingdom infantile spasms study (UKISS) compared a high-dose oral prednisolone protocol of 40 to 60 mg/day with IM synthetic ACTH, which showed spasms responder rates of 70% for prednisolone and 76% for ACTH with neither agents showing significant difference in EEG response rate at 14 days, P = 0.61 (18); however, further prospective studies are needed to confirm this finding.
Most institutions have their own treatment protocol for infantile spasms, with a wide variety of dose and duration (66–68). The most effective dose and duration of treatment with p-ACTH for remission of infantile spasms continues to be a controversial issue. Compared to prednisone, no major advantage was demonstrated by low doses of ACTH, whereas high doses were superior (58,59). High-dose p-ACTH at 60 IU/day or 150 IU/m2/day has produced excellent short-term response rates of 87% to 93% in prospective studies (58,63). In the only randomized, prospective comparison of p-ACTH, however, Hrachovy et al. (60) found no difference between high-dose and low-dose therapy. A prospective study using synthetic ACTH (62) by Yanagaki et al. compared very-low-dose (0.2 IU/kg/day) and low-dose (1 IU/kg/day) ACTH and found equivalent efficacy, with response and relapse rates comparable to those in other studies. Describing a stepwise increase in dosage, Heiskala et al. (65) demonstrated that while some patients can be controlled on lower doses of carboxymethylcellulose ACTH (3 IU/kg/day), others required high doses (12 IU/kg/day). Spasms were controlled initially in 65% of patients, but the relapse rate was high.
While some evidence supports high-dose ACTH over low-dose ACTH or oral steroids in cognitive outcome (9,11), the data are contradictory and not class I. Glaze et al. (55) found no difference between low-dose p-ACTH (20 to 30 IU/day) and prednisone (2 mg/kg/day). In a comparison of high-dose p-ACTH (110 IU/m2/day) and steroids, however, Lombroso (12) showed a higher rate of normal cognitive outcome in cryptogenic patients treated with ACTH than in those treated with prednisone alone (55% vs. 17%). In a retrospective comparison of different ACTH dosage regimens (69), Ito et al. also noted a positive correlation between dose and developmental outcome.
Although some data support high-dose ACTH as being more effective than low-dose ACTH, the precise dosage and duration are undetermined. The optimal dose may lie between 20 and 200 IU/m2/day. Doses of 400 IU/m2/day or higher are contraindicated because of a high incidence of life-threatening side effects (20,21,60,69).
There are some data suggesting that a good response to ACTH appears to be associated with better long-term outcome (24,70) in children with cryptogenic infantile spasms.
Based on these and other data, the current American Academy of Neurology Evidence–based guideline for the medical treatment of infantile spasms has concluded that low-dose ACTH should be considered for treatment of infantile spasms. ACTH or VGB may be useful for short-term treatment of infantile spasms, with ACTH considered preferentially over VGB. Hormonal therapy (ACTH or prednisolone) may be considered for use in preference to VGB in infants with cryptogenic infantile spasms, to possibly improve developmental outcome. A shorter lag time to treatment of infantile spasms with either hormonal therapy or VGB possibly improves long-term developmental outcomes (71).
Adverse Effects
ACTH and steroids, particularly at the high doses recommended for infantile spasms, can produce dangerous side effects. These are more frequent and more pronounced with ACTH. In prospective controlled trials, cushingoid features and extreme irritability were seen frequently; hypertension, while less common, was associated with higher doses (59–62). Vigilance is required for signs of sepsis, pneumonia, glucosuria, metabolic abnormalities involving the electrolytes calcium and phosphorus (72–74), and congestive heart failure (75,76). Of five deaths reported in prospective studies, at least two were directly attributable to ACTH (12,64).
Cerebral ventriculomegaly (59,77–81), which is not always reversible (55), can lead to subdural hematoma (82,83). The cause of the apparent cerebral atrophy is obscure, but its existence emphasizes the importance of diagnostic neuroimaging before initiation of ACTH.
Because hypothalamic–pituitary or adrenocortical dysfunction can result from ACTH therapy (84,85), morning levels of cortisol should be monitored during a taper and any medical stress treated with high-dose steroids (86). Treatment with ACTH or steroids can also be immunosuppressant and associated with infectious complications, perhaps as a result of impaired function of polymorphonuclear leukocytes (87). Both agents are therefore contraindicated in the face of serious bacterial or viral infection such as varicella or cytomegalovirus. Because of the high rate of fatal Pneumocystis pneumonia as an infectious complication of ACTH therapy (20,88–90), prophylaxis with trimethoprim–sulfamethoxazole, accompanied by folate supplementation and frequent blood counts, may be prudent in infants older than 2 months of age. In rare cases, ACTH also has been reported to exacerbate seizures (91,92).
Vigabatrin Versus Adrenocorticotropin
The 2004 American Academy of Neurology Practice Parameter for the medical treatment of infantile spasms has concluded that VGB is possibly an effective agent in the short-term treatment of infantile spasms (16). The 2012 American Academy of Neurology updated evidence-based guidelines for the medical treatment of infantile spasms concluded that while either ACTH (level B) or VGB (level C) could be offered for short-term treatment of infantile spasms, the evidence suggests that ACTH may be offered over VGB (level C) (71). Based on data from randomized controlled trials, from 23% to 65% of children treated with VGB achieve short-term remission of infantile spasms, with relapse rates of 4% to 20% (18,61,70,93,94).
Although VGB is thought to be particularly effective against infantile spasms associated with tuberous sclerosis (61,95,96) and is frequently advocated as a first-line therapy for this disorder, the data supporting this are retrospective (16). The mammalian target of rapamycin (mTOR) pathway is a key signaling pathway that is dysregulated in TSC. Animal studies have shown that VGB partially inhibited mTOR pathway activity and glial proliferation in the knockout mice in vitro as well as reduced mTOR pathway activation in cultured astrocytes from both knockout and control mice. This may account for the unique efficacy of VGB in TSC (97).
Limiting its use is the characteristic concentric constriction of visual fields. This effect does occur in childhood, and the risk may be cumulative with longer duration of therapy (98–102). The incidence in very young children is not known, and perimetric testing is often impossible in this group. Electrophysiologic studies in infants, although not as sensitive as perimetry, have confirmed VGB-associated abnormalities (101–104). In 22% to 32% of infantile spasms patients treated with VGB, reversible abnormal MRI signal intensity or restricted diffusion-weighted imaging in the thalamus, basal ganglia, dentate nucleus, and the brainstem have been reported. Although the clinical significance of these MRI changes is unknown, there is concern that the changes reflect a medication-related neurotoxic effect (105,106). VGB may have a place as a short-term treatment, although its long-term safety remains uncertain.
Other Agents in Infantile Spasms
Valproate (107,108), nitrazepam (109), pyridoxine (110), felbamate (111), intravenous immunoglobulin (112), topiramate (113), zonisamide (114), ganaxolone (115), levetiracetam (116), and the ketogenic diet (117,118) have been studied in small uncontrolled trials. However, there is insufficient evidence of efficacy and safety to recommend any of these therapies at this time (16,71).
Recommended Protocols for Adrenocorticotropin
In our center, the standardized therapy for new-onset infantile spasms, regardless of the etiology or lack thereof is to use VGB as first-line therapy. The reason for the use of VGB is its ease of use and relative lack of side effects compared to ACTH. We require complete resolution of spasms and hypsarrhythmia on EEG to continue VGB alone for a full 6-month course. Failing that, ACTH is begun as described below. This protocol has been shown to achieve seizure freedom in 96% of children so treated (119).
The optimal dose of ACTH required to enhance short-term response and long-term cognitive outcome is unknown; however, relatively high doses given early in the disease, accompanied by a second course in the event of relapse, appear warranted. The following high-dose ACTH regimen that has been used successfully in more than 500 children (58,63,120) is recommended (Table 67.2). A suggested protocol using Synacthen (cosyntropin or tetracosactide) based on the study done by Snead et al. (63) with 0.25 mg of Synacthen equivalent to 25 units of corticotropin is also included. The initial dose of ACTH is 150 IU/m2/day of ACTH gel, 80 IU/mL, intramuscularly in two divided doses for 1 week. In the second week, 75 IU/m2/day is given, followed by 75 IU/m2 every other day in the third week. Over the next 6 weeks, the dose is gradually tapered. The lot number of the ACTH gel is carefully recorded. Usually, a response is seen within the first 7 days; if within 2 weeks no response is noted or a steroid effect is evident, the lot is changed.
Table 67.2 Protocol for ACTH Therapy for Infantile Spasms
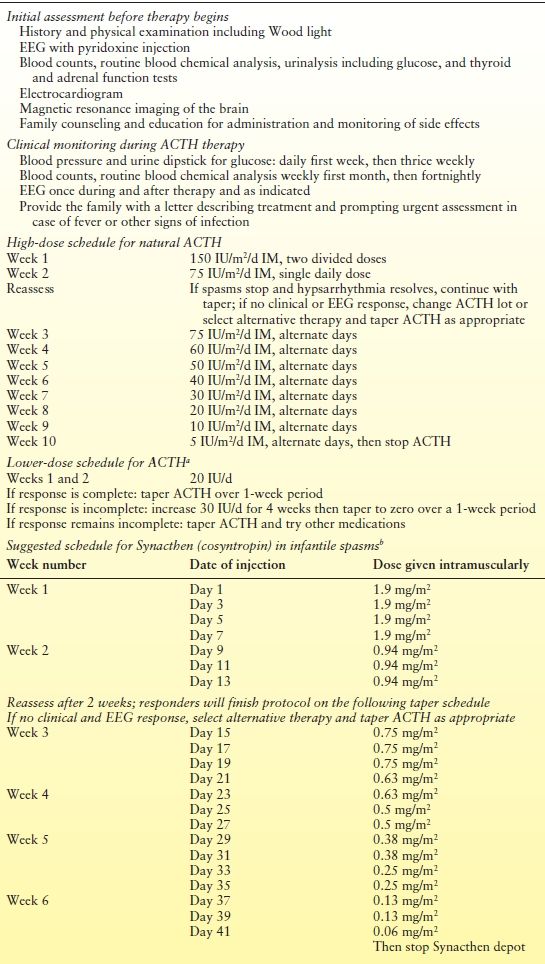
aBased on Hrachovy RA, Forst JD, Kellaway PR, et al. Double-blind study of ACTH vs. prednisone therapy in infantile spasms. J Pediatr. 1983;103:641–645 and Hrachovy RA, Frost JD, Glaze DG. High dose, long duration vs. low dose, short duration corticotropin therapy in infantile spasms. J Pediatr. 1994;124:803–806.
bThis 6-week Synacthen protocol developed and used at the Hospital for Sick Children, Toronto, Ontario.
A low-dose ACTH protocol based on two studies by Hrachovy et al. (59,60) showed clinical and EEG response rates of >40% in children with infantile spasms and provided evidence suggesting that low-dose ACTH is probably as effective as high-dose ACTH for the short-term treatment of infantile spasms. Patients receive 20 U/day for 2 weeks. If response was documented, the dose of ACTH was tapered to zero over a 1-week period. If a response was not documented, ACTH was increased to 30 U/day for 4 weeks and then tapered to zero during a 1-week period.
Once the decision is made to embark upon a course of ACTH therapy, the child is admitted to a day care unit for initiation therapy. Parents are taught to administer the injection, measure urine glucose three times daily with Chemstix, and recognize spasms so as to keep an accurate seizure calendar. Any diagnostic workup indicated by clinical circumstances is also performed, including screening for occult infections. Before ACTH is started, an endocrine profile, complete blood count, urinalysis, electrolyte panel, baseline renal function tests, and calcium, phosphorus, and serum glucose levels are obtained. Blood pressure is measured and an electrocardiogram performed. The drug is not given if any of these studies show abnormal results. Diagnostic neuroimaging is indicated before initiation of ACTH or steroids because of the association with ventriculomegaly.
Blood pressure must be measured daily at home during the first week and three times weekly thereafter. Control of hypertension is attempted with salt restriction and amlodipine therapy rather than discontinuation of ACTH. The patient is monitored in the outpatient clinic weekly for the first month and then biweekly, with appropriate blood work at each visit. Waking and sleeping EEG patterns are obtained during and after the start of ACTH to assess treatment response. Because a response is usually noted within a week or two of initiating ACTH (57–59), positive results are suggested when properly trained parents report no seizures in a child whose waking and sleeping EEG patterns are normal.
If relapse occurs, the dose may be increased to the previously effective dose for 2 weeks and another tapering begun. If seizures continue, the dose may be increased to 150 IU/m2/day and the regimen restarted.
Recommended Protocols for Prednisone and Prednisolone
If prednisone is chosen because of its oral formulation and lower incidence of serious side effects, the pretreatment laboratory evaluation described earlier is performed. The initial dose is 3 mg/kg/day in four divided doses for 2 weeks, followed by a 10-week taper (115). A multiple daily dose regimen of high-dose ACTH therapy is recommended to produce sustained elevations of plasma cortisol (57,63).
The UKISS by Lux et al. (18) used the following high-dose oral prednisolone regimen: initial dose is 10 mg four times a day for 2 weeks, increasing to 20 mg three times a day after 1 week if spasms continued, followed by taper of 10 mg every 5 days or, if on the higher-dose treatment, 40 mg daily, then 20 mg, then 10 mg for 5-day periods.
OTHER SEIZURE DISORDERS
Ohtahara and Lennox–Gastaut syndromes are believed to represent earlier and later manifestations, respectively, of a spectrum of infantile epileptic encephalopathies that include infantile spasms (121–124). These conditions respond poorly to traditional anticonvulsant drug therapies but are sometimes improved by the antiepileptic drugs used in infantile spasms: ACTH, steroids, benzodiazepines, and valproic acid. ACTH of steroids also may be beneficial in Landau–Kleffner syndrome.
Ohtahara Syndrome
Also known as early infantile epileptic encephalopathy, Ohtahara syndrome is characterized by spasms beginning within the first 3 months of life associated with persistent burst suppression on the EEG in all stages of the sleep–wake cycle (121). Despite reports of improvement after ACTH (121,125), VGB (126), and zonisamide (121), the long-term prognosis usually is unchanged by any treatment (121,123) and involves high mortality and severely handicapped survivors. If used, ACTH should be administered as described for infantile spasms.
Lennox–Gastaut Syndrome and Other Myoclonic Disorders
ACTH and steroids have been found useful in younger children with various combinations of severe and intractable seizures, particularly atypical absence, myoclonic, tonic, and atonic seizures (1,38,120,127–132). This group includes patients with Lennox–Gastaut syndrome, a disorder characterized by mental retardation, generalized slow spike-and-wave discharges, intractable atypical absence, myoclonus, and frequent ictal falls. Several uncontrolled, retrospective studies suggest that ACTH is superior to oral steroids against these seizure types (121,127,129,130), and the regimen described in this chapter for ACTH or prednisone is recommended. Nevertheless, ACTH and steroids should be reserved for the most severe and intractable disease. Usually, the best result is temporary relief, because 70% to 90% of patients with multiple seizure types suffer a relapse during the ACTH taper (120).
In another age-dependent disorder first described by Doose (133), myoclonic astatic seizures begin between 7 months and 6 years of age in a previously normal child and are associated with generalized discharges on the EEG (134). This disorder is resistant to most conventional antiepileptic drugs; however, a retrospective study has reported response to the ketogenic diet, ACTH, and ethosuximide (134).
Landau–Kleffner Syndrome and Related Disorders
Described in 1957 (135), Landau–Kleffner syndrome, also known as acquired epileptic aphasia, is characterized by regression in receptive and expressive language associated with epileptic seizures. The usual presentation occurs between the ages of 2 and 8 years. Clinical seizures may precede, be coincident with, or develop after the onset of language deterioration, and up to 25% of patients with language loss and epileptiform EEG patterns never experience clinical seizures (136,137). Behavioral disturbances are frequent, ranging from hyperactivity and aggressiveness to autism and global cognitive deterioration. Some children display sustained agnosia and mutism; others show a waxing and waning course that parallels the EEG changes; still others demonstrate spontaneous resolution (137). The EEG typically shows 1- to 3-Hz high-amplitude spike and slow waves; these may be unilateral, bilateral, unifocal, or multifocal but often include the temporal region, with or without parietal and occipital involvement, and are activated in sleep (138).
Valproate and benzodiazepines may control the syndrome’s clinical seizures but have only a partial and transient effect on the EEG abnormalities (10,139). In 1974, McKinney and McGreal (140) described the beneficial effect of ACTH on the characteristic seizures, language regression, and behavioral change. Since then, although no controlled prospective trials of ACTH or steroids have been published, case reports and retrospective series have demonstrated improvements in seizure control and language in children treated with varying ACTH or corticosteroid regimens (10,137–139,141–143).
The use of ACTH or corticosteroids in patients with Landau–Kleffner syndrome appears justified; however, further study of dose and duration of therapy is warranted, as is exploration of new anticonvulsants. High-dose ACTH or prednisone, as described in this chapter for infantile spasms, may be useful, with a longer tapering schedule and concomitant use of valproic acid.
References
1. Klein R, Livingston S. The effect of adrencorticotrophic hormone in epilepsy. J Pediatr. 1950;37:733–742.
2. Sorel L, Dusaucy-Bauloye A. A propos de cas d’hypsarythmia de Gibbs: son traitement spectulaire par l’ACTH. Acta Neurol Belg. 1958;58:130–141.
3. Dumermuth G. Über die Blitz-Nick-Salaam-Krämpfe und ihre Behandlung mit ACTH und Hydrocortison. Mitt Helv Pediatr Acta. 1959;14:250–270.
4. Gastaut H, Salfiel J, Raybaud C, et al. A propos du traitement par l’ACTH des encéphalites myoclonique de la première enfance avec majeure (hypsarythmie). Pediatrie. 1959;14:35–45.
5. Low N. Infantile spasms with mental retardation. I: Treatment with cortisone and adrenocorticotropin. Pediatrics. 1958;22:1165–1169.
6. McQuarrie I, Anderson JA, Ziegler RR. Observations on the antagonistic effects of posterior pituitary and cortico-adrenal hormones in the epileptic subject. J Clin Endocrinol Metab. 1942;2:406–410.
7. Stamps FW, Gibbs EL, Rosenthal IM, et al. Treatment of hypsarrhythmia with ACTH. JAMA. 1959;171:408–411.
8. Koo B, Hwang P, Logan W. Infantile spasms: outcome and prognostic factors of cryptogenic and symptomatic groups. Neurology. 1993;43:2322–2327.
9. Lerman P, Kivity S. The efficacy of corticotropin in primary infantile spasms. J Pediatr. 1982;101:294–296.
10. Marescaux C, Hirsch E, Finck S, et al. Landau–Kleffner syndrome: a pharmacologic study of five cases. Epilepsia. 1990;31:768–777.
11. Sher PK, Sheikh MR. Therapeutic efficacy of ACTH in symptomatic infantile spasms with hypsarrhythmia. Pediatr Neurol. 1993;9:451–456.
12. Lombroso C. A prospective study of infantile spasms: clinical and therapeutic correlations. Epilepsia. 1983;24:135–158.
13. Kivity S, Lerman P, Ariel R, et al. Long-term cognitive outcomes of a cohort of children with cryptogenic infantile spasms treated with high-dose adrenocorticotropic hormone. Epilepsia. 2004;45:255–262.
14. West W. On a peculiar form of infantile convulsions. Lancet. 1841;1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








