To address the above issues, the International League Against Epilepsy (ILAE) issued a commission report proposing a new outcome classification scheme (Table 90.2). Completely seizure-free patients are classified separately; seizures are quantified in each category and compared to a well-defined baseline frequency, and results can be easily compared to AED trials. To date, only one study (26) compared both systems in its outcome assessment and found similar results at the last available follow-up.
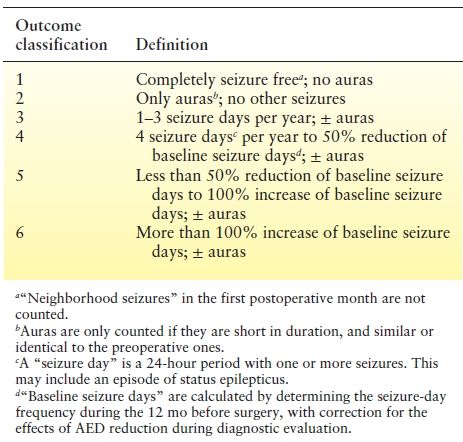
Table 90.2 Proposal for a New Classification of Outcome with Respect to Epileptic Seizures
Some centers reported their outcomes using internally validated scoring systems (24,31,32). Others chose a prespecified period of seizure freedom—usually 12 to 24 months—as reflecting a favorable outcome (4,33,34).
This wide variation in outcome measures is only one of many pitfalls complicating the interpretation and comparison of the results among different surgical series. Other issues comprise (i) including patients with heterogeneous disease pathologies and surgeries in the same study limiting the validity of the results for any one group; (ii) using cross-sectional methods of analysis, which, by definition, are inaccurate in analyzing longitudinal dynamic time-dependent outcomes like postoperative seizure freedom; and (iii) the lack of studies comparing the usefulness of various surgical diagnostic (e.g., invasive subdural vs. depth recordings) or treatment techniques (e.g., resective vs. radiosurgery vs. thermo- or laser ablation). The final limitation of our current outcomes understanding remains our inability to “individually” predict the chances of success for potential surgical candidates.
TEMPORAL LOBE SURGERY
Rate and Stability of Postoperative Seizure Freedom
TL is the most common type of resective epilepsy surgery performed. One randomized controlled trial (1) showed that only two intractable epilepsy patients need to be treated surgically for one patient to become free of disabling seizures. Table 90.3 summarizes the seizure outcomes of most major centers, showing relatively comparable results with about two-thirds of the patients becoming seizure free postoperatively, compared to 5% to 8% with medical therapy. More than 50% of patients remain seizure free beyond 10 years after anterior temporal lobectomy (ATL) reflecting a sustained benefit (3,5,35–38).
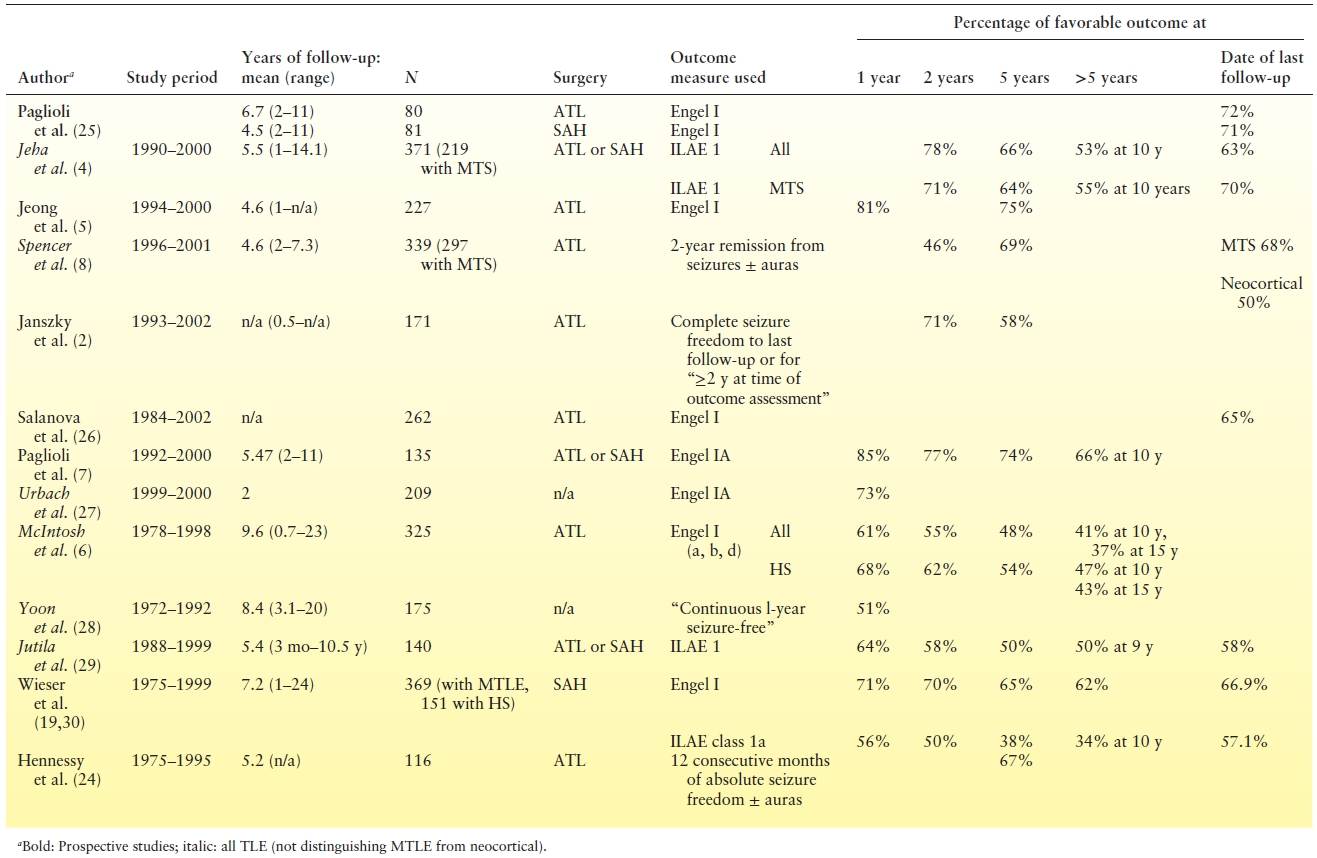
Table 90.3 Surgical Outcome in Major Studies Evaluating Pure Cohorts of Patients with Hippocampal Sclerosis
If a patient is seizure free at 1 year postoperatively, the likelihood of remaining seizure free is 87% to 90% at 2 years, 74% to 82% at 5 years, and 67% to 71% at 10 years (3,5, 35–39). If a patient is seizure free for 2 years postoperatively, chances of seizure freedom increase up to 95% at 5 years, 82% at 10 years, and 68% at 15 years (8,40). So, seizure freedom for 2 years might be a better predictor of long-term outcome, although both the 1-year and the 2-year conditions correlate fairly well with subsequent seizure-free status.
In surgical failures, more than half of seizure recurrences start within 6 postoperative months, and more than 95% recur within 2 to 5 postoperative years (8,41). There is therefore an initial phase of steep recurrence, followed by a relapse rate of 2% to 5% per year for 5 years with subsequent more stable seizure freedom (6,8,36). Recent data suggest that prognostic factors affecting those two phases of recurrence are distinct (8,28,29,33,42,43), possibly reflecting different mechanisms for early versus late relapses. “Early recurrences” occurring within 6 to 12 months of surgery may be due to incomplete removal of the initial epileptogenic zone, whereas later relapses may reflect an underlying diffuse epileptogenicity or progression of an “age-dependent” etiology such as mesial temporal sclerosis (2,4,28,29,36,39,44,45). The main implication of this mechanistic perspective is the idea that improving seizure outcomes necessitates both optimal localization and resection of the epileptic focus AND strategies aimed at preventing future epileptogenesis.
The counterpart of late seizure relapses also exists. In the “running-down” phenomenon, defined as the late remission of postsurgical seizures and occurring in 3.2% to 20% of TLE surgery cases, the frequency of seizures during the running-down interval may be up to several per month, but a seizure-free state is usually achieved within 2 years (44,46). The most accepted explanation for this phenomenon is a dekindling effect, the converse process to secondary epileptogenesis, where the induced synaptic dysfunction gradually declines in the surrounding epileptogenic cortex after pacemaker resection, and eventually “runs itself down” (46).
Predictors of Recurrence
Clinical Variables and Seizure Outcome
Age at Onset of Epilepsy.
Patients with an earlier age at onset of epilepsy (usually <5 years) or at the time of the initial neurologic insult may be up to three times more likely to have a favorable postoperative outcome (5,37). However, some investigators proposed that this variable actually predicts hippocampal sclerosis (HS), which is the actual good prognostic indicator (34,47). This hypothesis is supported by the observation that those patients were more likely to have features typical of HS such as unilateral hippocampal atrophy on MRI (48) and focal ictal electroencephalogram (EEG) with predominantly partial seizures (37) and by the fact that age at onset per se was of no prognostic value in studies evaluating pure cohorts of HS (34,47) or controlling for pathology (4,6,8).
Duration of Epilepsy.
A long history of seizures correlated with worse outcome in multiple studies on univariate analysis (24,41,44). In some of those same cohorts, this influence disappeared when multivariate analysis was performed adjusting for other more solid indicators of outcome (6,7). Furthermore, many more recent studies found no correlation of epilepsy duration with outcome (4–6,34,47,49). Various hypotheses have been proposed to explain those findings, including secondary epileptogenesis occurring with a long seizure history, varying degrees of maturation of different epileptogenic foci, and the increased development of generalized seizures with longer epilepsy duration (44).
Age at Surgery.
Most studies found no correlation between age at surgery and seizure outcome (5,6,8,34), although one longitudinal study in HS patients found that patients who were ≤24 years old at surgery were about four times more likely to be seizure free at 5 postoperative years when compared to the older surgical group (36 years or older) (7). Few other studies found similar results (44).
One should note here that successful and safe ATLs have been performed in the elderly (>50 years old), with few reports suggesting slightly lower chances of seizure freedom albeit without increased risks of neuropsychological deficits (44). Therefore, older age by itself should not be a deterrent from surgery.
Absence of Secondarily Generalized Tonic–Clonic Seizures.
Only 57% of mesial TLE patients with secondarily generalized tonic–clonic seizures (SGTCS) achieved a 1-year remission compared to 80% remission rate in those who had only partial seizures in one study (34). Patients who had no generalized tonic–clonic seizures (GTCS) were 2.2 times more likely to be seizure free 5 years after surgery in another study (7). This effect may be most significant when GTCS are frequent (more than two per year) and occurring within 3 years of surgery (6). The prognostic significance of SGTCS was confirmed in a prospective multicenter trial (4).
The occurrence of SGTCS in TLE correlates with more extensive HS, multifocal irritative areas, and extended positron emission tomography (PET) hypometabolism suggesting a diffuse potential epileptogenic zone with worse expected surgical outcome (44).
Other.
Clinical variables where some studies suggested a favorable prognostic significance include low baseline seizure frequency and a history of febrile seizures. This, however, was not consistently confirmed. No correlation between occurrence of auras and outcome was proven (8).
Imaging Variables and Seizure Outcome
Magnetic Resonance Imaging.
A consistently identified favorable outcome predictor has been the presence of a unilateral temporal lobe abnormality on MRI (4,44,47). Patients with MRI evidence of unilateral HS had a 54% chance of seizure freedom at 10 years after ATL compared to 18% if MRIs were normal in one longitudinal study (6). However, recent data suggest that such a favorable prognostic significance is actually conferred by ANY unilateral temporal MRI lesion, and not necessarily by HS, especially with concordant ictal and interictal EEG findings (44).
Although a normal MRI was traditionally considered an automatic correlate to surgical failure (40,41), recent data have actually shown seizure-freedom rates of up to 41% to 48% as long as 8 years after ATL (18,50–54). While some data suggest that these patients may actually have “MRI-negative” or undetected HS (44), other studies concluded that most cases of normal-appearing hippocampi on high-resolution MRI have neocortical TLE since they had less febrile seizures, more delta rhythms at ictal onset, and more extensive lateral neocortical changes on PET with surgical outcomes still comparable to those of HS obvious in MRI (51,53). It should be emphasized, though, that surgery was successful in nonlesional patients typically when performed in context of concordant EEG and PET data (18,51,53). “Normal” MRIs correlating with bad outcomes in older studies using lower quality imaging may have included patients with extratemporal or contralateral pathology, findings that would currently exclude viable surgical options (18,51,53). Lack of SGTCS and low preoperative seizure frequency correlate with more favorable surgical outcomes in nonlesional TLE, similar to lesional TLE (18).
Bilateral MRI lesions, including grossly bilateral HS, reflect multiple potentially epileptogenic foci and correlate with a worse surgical outcome: 58% seizure free at 2 years compared to 78% when compared to unilateral lesions or even normal MRI (8,44). Subtle hippocampal asymmetries only detected using volumetric analyses were less predictive of outcome (44).
Nuclear Imaging.
Unilateral temporal hypometabolism on FDG-PET is a good predictor of seizure freedom in patients with mesial TLE, independent of pathologic findings and regardless of whether the MRI is normal (55). In a recent review of the literature, Casse (55,56) found that 86% of patients with unilateral temporal hypometabolism ipsilateral to the side of surgery had a good outcome as defined by more than 90% reduction in seizure frequency or Engel class I or II, with those chances slightly reduced to 82% if the MRI was normal. This number significantly dropped to 62% when PET was normal and to 50% when it showed bitemporal hypometabolism (56). With extratemporal hypometabolism, chances of seizure freedom are even worse: Complete seizure freedom at last follow-up (mean 6.1 years) was seen in 45% of patients with extratemporal cortical hypometabolism confined to the ipsilateral hemisphere and only 22% with contralateral cortical hypometabolism (57).
Abundant data support the usefulness of ictal SPECT in localizing the epileptogenic zone in TLE, with 70% to 100% of ictal SPECTs being correctly localizing and only 0% to 7% incorrectly localizing (44). However, while the prognostic value of such localized SPECT findings is clear in extratemporal or poorly localized nonlesional temporal epilepsy (58,59), its role in clear lesional TLE cases is less defined. In a recent analysis of patients with unilateral HS visible on MRI, surgical outcome was not influenced by contralateral increased flow on ictal SPECT (60). One hypothesis is that due to their low temporal resolution, ictal SPECT hyperperfusion patterns often contain both the ictal-onset zone and propagation pathways. These patterns often have a multilobulated “hourglass” appearance with the largest and most intense hyperperfusion cluster often representing ictal propagation and not necessarily requiring resection to render a patient seizure free (61,62). Results for interictal SPECT suggest that it is relatively poor at localizing the seizure focus (44).
Neurophysiologic Variables and Seizure Outcome
Noninvasive EEG.
Focal interictal EEG predicts a favorable outcome when lateralized to the side of surgery or when highly localized to the resected temporal lobe. Patients whose interictal EEGs showed ≥90% predominance on the operated-on side had an 80% chance of complete seizure freedom after a mean 5.5 years of follow-up versus 54% in those with lesser degrees of lateralization in one prospective study (5). In general, interictal evidence of a diffuse irritative zone predicts a worse outcome: Postoperative seizure freedom is worse when interictal spiking was posterior temporal, extratemporal, or bitemporal (44). Posterior temporal and extratemporal spiking in patients with pathologically confirmed HS may reflect diffuse epileptogenicity or “dual pathology” with associated neocortical epileptogenic zones, thereby explaining the associated worse prognosis (34,63). However, prognostic implications of bitemporal interictal spiking on surface EEG deserve more careful consideration, as it does not automatically preclude postoperative seizure freedom. One study found that if ≥90% of surface interictal bitemporal spikes arise from one temporal lobe, excellent outcome is possible (92% seizure free in the second postoperative year vs. 50% if <90% lateralization), and further evaluation with depth EEG electrodes may not even be indicated (64). With a unilateral MRI temporal lesion, and with lateralizing WADA or neuropsychiatric testing, up to 64% of patients with bilateral interictal spikes achieved complete seizure freedom at ≥1 year postoperatively when seizure onset was strictly unilateral on invasive evaluation (65). Other findings consistent with unilateral HS, such as a history of febrile seizures or early onset of epilepsy (prior to age 3 to 6 years), also correlated with favorable outcome in patients with bitemporal interictal spikes suggesting that contralateral spiking may simply be spread from a surgically treatable hippocampus (44). However, if the MRI is normal or shows widespread abnormalities, then seizure recurrence is the rule as either an extratemporal focus spreading to both temporal lobes or bitemporal epilepsy becomes more likely (65).
Similar concepts apply to the prognostic value of ictal EEG. Again, focal or anterior ictal EEG correlates with a more favorable outcome, and patients who had bitemporal ictal onsets on surface EEG still achieved seizure-freedom rates of up to 64% at 1 postoperative year if seizures were exclusively unilateral with depth recordings and imaging or neuropsychological testing were also consistent with unilateral temporal dysfunction (44,65).
Intracranial EEG.
Depth electrode evaluations have traditionally been used to clarify lateralization of the epileptogenic zone in patients with suspected bitemporal or falsely lateralized TLE, whereas subdural recordings and stereoelectroencephalography (SEEG) are useful in neocortical epilepsy for extraoperative functional mapping and definition of the extent of the epileptogenic zone. Those modalities are therefore reserved for patients with a poorly defined epileptogenic zone, which may explain poorer outcomes seen in cases that required invasive recordings preoperatively compared to those that did not (8,38,44,66). Outcomes are particularly worse in patients who had prior temporal lobe resections (67). Yet, specific findings obtained with such invasive evaluations may provide useful prognostic information. During depth recordings, more favorable outcomes are seen with exclusively unilateral seizure onset and ictal spiking as opposed to low-voltage fast activity, electrodecrement, or any other rhythmic sustained activity at seizure onset, whereas evolution into distinct contralateral electrographic seizures lowered seizure freedom from 84% to 47% at 1 postoperative year (44). Short interhemispheric propagation times ranging from <1 second to <8 second, a short duration between EEG and clinical seizure onset, and diffuse or posterior temporal onset as opposed to anterior and/or middle basal temporal ictal onset have all been also identified as predictors of seizure recurrence after surgery (44).
Surgical Technique and Seizure Outcome
Similar seizure-freedom rates have been observed with selective amygdalohippocampectomy and anterior TL (8,68,69). Many studies failed to correlate the extent of temporal resection (37), the extent of hippocampal resection (38), or having a mesial versus neocortical resection (8,70) to outcome. Those studies, however, did not evaluate patients with mesial TLE separately. In the presence of unilateral mesial TLE with HS, the extent of mesial resection becomes a more significant predictor of postoperative seizure freedom (44). In a prospective, randomized, blinded clinical trial, Wyler et al. (71) found that only 38% of patients in whom the hippocampal resection was limited posteriorly by the projection of the lateral mesencephalic sulcus (partial hippocampectomy) were seizure free at 1 year, compared to 69% of those in whom the hippocampus was removed further, to the level of the superior colliculus (almost complete resection). The amount of amygdala that must be resected to achieve seizure freedom is unclear, although one study found no correlation between residual amygdalar tissue and surgical outcome (72). The ideal extent of lateral temporal resection also remains to be defined with conflicting data currently available (44).
In the presence of a well-circumscribed lesion, such as a tumor or a vascular malformation, a lesionectomy plus resection of the nearby adjacent cortex might suffice unless there is associated hippocampal atrophy. In such cases of dual pathology, complete seizure freedom after a mean follow-up of 37 months was lowered from 73% with lesionectomy plus mesial temporal resection to 20% with mesial temporal resection alone and 12.5% with lesionectomy alone (44,73,74).
Etiology, Pathology, and Seizure Outcome
When pathologic findings in the resected temporal lobe were restricted to nonspecific gliosis, poor short- and long-term outcomes have consistently been observed (44). In a longitudinal study of 371 ATL patients, 44% of cases who only had gliosis were seizure free 8 years after surgery, compared to 64% if a specific pathologic diagnosis was identified (8). However, once a specific pathologic abnormality is identified, it is not entirely clear that its nature is relevant for seizure outcomes. While the older literature has suggested more favorable outcomes with HS, seizure-freedom rates were similar between HS and other types of lesions in many recent (6,8) or prospective studies (4,70,75). One hypothesis is that outcome depends not only on the presence of HS but also on its severity: Worse disease may predict better outcome. One group found that 84% of patients with classical HS, as defined by neuronal loss and sclerosis in CA1, CA4, and the granule cells of the dentate gyrus, achieved at least 95% seizure reduction at last follow-up, compared to only 29% of those where cell loss was restricted to the dentate gyrus and/or CA4 (53). Another group also found that rates of Engel class I outcomes at last follow-up increased from 60% to 76% to 89% as the pathologic severity of HS ranged from mild to moderate to severe (26).
FRONTAL LOBE SURGERY
Rate and Stability of Postoperative Seizure Freedom
Frontal lobectomy (FL) accounts for 6% to 30% of all epilepsy surgeries and represents the second most common procedure performed to treat intractable focal epilepsy after TL. However, reported seizure-freedom rates with frontal resections have varied from 13% to 80% (11,15,17,54, 76–82), suggesting, in general, significantly lower success rates than those observed with temporal resections. Only few studies evaluated seizure freedom after FL longitudinally and can therefore provide useful information related to rate and stability of seizure outcome over time (11,77,83). In a retrospective study evaluating 97 adults who underwent resective FL surgery between 1991 and 2005, Elsharkawy et al. (77) found that the probability of an Engel class I outcome was 54.6% at 6 months, 49.5% at 2 years, 47% at 5 years, and 41.9% at 10 years. In a study reviewing patients operated at Cleveland Clinic between 1995 and 2003, and using a stricter “favorable outcome” definition (complete seizure freedom since surgery), we had previously identified a seizure-freedom rate of 55.7% at 1 postoperative year, 45.1% at 3 years, and 30.1% at 5 years and beyond (11). Outcomes were somewhat more favorable, closer to the 40% seizure-free range in a more recent series (20). Eighty percent of seizure recurrences occur within the first 6 postoperative months, and although late remissions and relapses may occur, those are usually rare (11). One study showed that although a postoperative reduction in seizure frequency often occurred in patients who failed to become completely seizure free after surgery, this improvement was sustained until the last follow-up in only 35%, with seizure frequencies eventually returning to preoperative levels in the remainder (11). The running-down phenomenon previously described may occur following FL, but at a rate of <15%, also significantly less than that seen after TL (77).
Similar to TL, however, seizure freedom at 6 months to 2 postoperative years seems to be a very good predictor of a long-term seizure-free state. If a patient is seizure free at 2-year follow-up, the probability of remaining seizure free up to 10 years may increase up to 86% (77).
Predictors of Seizure Recurrence
Mechanistically, proposed hypotheses to explain the generally lower rates of seizure freedom following FL include (i) difficulty localizing the epileptogenic zone with EEG data secondary to rapid ictal spread through the frontal lobe, (ii) difficulty achieving a complete surgical resection secondary to proximity of functional/eloquent cortex, and (iii) a preponderance of cortical dysplasia, often invisible on MRI, as the epilepsy etiology in the frontal lobe as opposed to clearly localized HS in the temporal lobe (14,79,84). Practically, identified predictors of postoperative seizure recurrence have included incomplete resection of the epileptic lesion (11,22,77,85,86), the need to perform an invasive EEG evaluation (11,77), the occurrence of acute postoperative seizures (11), the persistence of auras postoperatively (11,77), a history of febrile seizures (79), predominantly generalized or poorly localized ictal EEG patterns on surface EEG prior to surgery—especially in the adult population (11,13,17,77) —and the lack of a distinct single MRI lesion (11,13,77,85). Of all the above prognostic indicators, the two most consistently reported and strongly predictive of postoperative seizure freedom are the presence of an MRI lesion and completeness of resection (Figs. 90.1 and 90.2). A short epilepsy duration (<5 years) is associated with significantly improved outcomes, regardless if patients are lesional or not, a finding that highlights the urgency and importance of early surgical referral in patients with FLE (20).
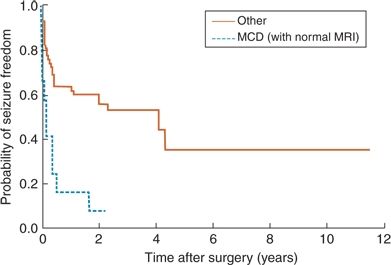
Figure 90.1. Survival curve illustrating lower long-term rates of seizure freedom in patients with normal MRI as opposed to lesional cases following frontal lobe resection. (Adapted from Jeha LE, Najm I, Bingaman W, et al. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007; 130(Pt 2):574–584.)
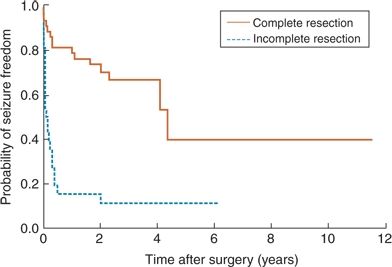
Figure 90.2. Survival curve illustrating lower long-term rates of seizure freedom in patients with incomplete resection as opposed to complete resections following frontal lobe resection. (Adapted from Jeha LE, Najm I, Bingaman W, et al. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007; 130(Pt 2): 574–584.)
MRI and Seizure Outcome
A normal MRI in a patient undergoing FL has consistently been found to predict a worse outcome. Twenty-five percent of the patients with negative MRI studies and 67% of those with neuroimaging abnormalities restricted to the frontal lobe were seizure free at a minimum duration of follow-up of 1 year in one study (87). A focal MRI abnormality was the only variable significantly associated with a favorable surgical outcome in another report (88). Only 41% of nonlesional FLE patients had an excellent outcome versus 72% when MRI abnormality was present in yet another retrospective analysis (79). Most such “nonlesional” FLE cases are thought to have an underlying malformation of cortical development (MCD) (13,85). In one series, all patients with normal MRI and pathologically proven MCD had recurrent seizures by 3 postoperative years (11). Knowing that milder forms of MCD such as microdysgenesis, cortical dyslamination, or focal MCD are often missed, even on high-resolution MRI may explain why one cannot “see” the extent of the epileptogenic tissue in those MRI-negative MCD cases making adequate surgical treatment harder. The particular pathologic MCD substrate in fact was critical in determining surgical outcome in another more recent series with the worst outcomes in type I MCD (19).
Techniques such as ictal SPECT imaging, FDG-PET, and subdural grid or SEEG monitoring are often used to better localize the epileptogenic zone in nonlesional FLE cases. A study reporting on 193 patients with neocortical focal epilepsy (including 61 with FLE) showed that correct localization by FDG-PET was an independent predictor of a good outcome (13), and other case reports highlighted the usefulness of ictal SPECT in identifying a potential epileptogenic zone in nonlesional FLE (84). A recent analysis, however, found that while MRI, PET, and ictal SPECT all had good positive predictive values with correspondingly acceptable negative predictive values in correlating with the ictal-onset zone as later defined by invasive EEG recording, there was no significant relationship between the diagnostic accuracy of any of these modalities and surgical outcome, with the exception of MRI (P = 0.029) (15). So, the translation of “accurate” and “correct localization” of epileptic foci using either PET or SPECT into actual improvements in seizure outcome for nonlesional FLE has not always been consistently reproducible. The interpretation of the role of intracranial EEG monitoring is another delicate issue. In a cross-sectional study of 51 nonlesional, mostly FLE, cases operated on between 1992 and 2002, Wetjen et al. (76) found that 35.7% of the 28 patients who eventually underwent a focal resection after intracranial EEG recording became seizure free with high-frequency oscillations at ictal onset being predictive of seizure freedom. Since this study’s patient population included cases operated on prior to the advent of FLAIR imaging and other high-resolution neuroimaging techniques, an unknown proportion of its cases may have had subtle structural abnormalities, which potentially could have been detected using current imaging modalities. A longitudinal study of FLE patients imaged and operated on more recently found that 66% were seizure free at 1 postoperative year, and 44% (95% CI = 39 to 49) at 5 years and beyond, including nonlesional cases (20). In summary, while nonlesional FLE cases seem to be as a whole less than ideal surgical candidates for resective epilepsy surgery, efforts to identify the specific subgroup that might benefit from surgery while pursuing nonsurgical treatment options for the rest are still required. Early referral for a presurgical evaluation is essential (20).
Any extrafrontal MRI abnormality also confers a poor prognosis. Favorable outcomes occurred in either none of the patients with multilobar MRI abnormalities (87) or at best in 10% to 14% (11,89). Tumors, well-circumscribed pathologies, usually have the best outcome with up to 62% seizure free at last follow-up in one report (11) and 65% Engel class I or II at last follow-up in another series (14).
European series from centers using stereo–EEG evaluations report more favorable outcomes in nonlesional extratemporal epilepsy (90,91), highlighting the importance of adequately developing a preimplantation hypothesis prior to exploration of these patients for resective surgery.
Extent of Resection and Seizure Outcome
Complete resection of the epileptogenic lesion has consistently been found to predict seizure freedom. In one report, of patients who had complete removal of their epileptogenic lesions, 81% were seizure free at 1 year and 66% at 3 years, compared to 13% and 11%, respectively, of those who did not (11). Complete removal of neuroimaging abnormalities (76,85,92) and abolition of residual ECoG spiking (93) or seizures (94) have also been linked with the most favorable outcomes following FLE surgery. Major challenges that hinder a complete resection in all cases include frequent proximity or overlap with eloquent cortex and difficulties identifying the true edges of the “abnormal” tissue in MCD cases where the MRI-visible portion of the dysplasia may be surrounded by microscopically abnormal tissue that seems normal on imaging (11).
In summary, while the rates of seizure freedom are low, in general, following frontal resections, very successful seizure outcomes are possible in a selected group of patients, mainly those with a clear MRI lesion that is completely resectable.
POSTERIOR CORTEX SURGERY
Rate and Stability of Postoperative Seizure Freedom
Resections in the posterior cortex represent <10% of all epilepsy surgeries, with reported postoperative seizure- freedom rates varying from 25% to 90% (12,85,95–98). In a longitudinal analysis of a cohort of posterior cortex resections, the estimated chance of seizure freedom was 73.1% at 6 postoperative months, 68.5% at 1 year, 65.8% between 2 and 5 years, and 54.8% at 6 years and beyond. The median timing of recurrence was 2.0 months with 75% of the seizure recurrences occurring by 6.4 months, and late recurrences were rare with the latest being at 74 months (12). Similar rates of seizure freedom have been reported in another longitudinal analysis of 154 adult patients who underwent various types of extratemporal resections (about 40% frontal and the remaining being posterior cortex surgeries), with an Engel class I at 2 postoperative years being correlated with an 88% chance of remaining seizure free 14 years after surgery (83). These findings suggest that in posterior cortex resections, we can expect an initial rate of seizure recurrence that is as fast as following FL, allowing a relatively early identification of surgical failures, but with a more optimistic long-term outlook with late seizure-free rates comparable to those following temporal resections. Figure 90.3 illustrates the longitudinal rates of seizure freedom in posterior quadrant resections in a cohort evaluated at Cleveland Clinic recently.
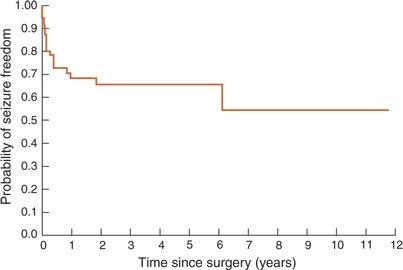
Figure 90.3. Survival curve illustrating long-term rates of seizure freedom following posterior quadrant surgery. (Adapted from Jehi LE, O’Dwyer R, Najm I, et al. A longitudinal study of surgical outcome and its determinants following posterior cortex epilepsy surgery. Epilepsia. 2009;50(9):2040–2052.)
Predictors of Seizure Recurrence
Patients with well-circumscribed focal lesions (tumors or MRI-visible MCD), who have more extensive resections (lobectomies or multilobar resections as opposed to lesionectomies), no preoperative evidence of extralobar epileptogenicity extending to the ipsilateral temporal lobe (temporal spiking or auditory auras), and no postoperative evidence of residual epileptogenicity (spiking on 6 months postoperative EEG) had the most favorable outlook in most series of posterior cortex resections (12,83,85,95–98).Other less consistently reported predictors of seizure freedom include lateralizing seizure semiology (95), focal ictal EEG (99), and shorter epilepsy duration (100).
In a series of 57 patients with posterior cortex resections, only a quarter of patients with either a tumor or lesional MCD had postoperative seizure recurrence, as opposed to more than half of the patients who had other pathologies after a mean follow-up of 3.3 years (12). Completeness of resection of such epileptogenic lesions was identified, among others, by Barba et al. (99) in 2005 to be the strongest predictor of postoperative seizure freedom. The challenge though is that while it is easily understood that larger resections have a better chance of achieving seizure freedom, this may not always be possible secondary to risks of injury to eloquent cortex, especially in the dominant hemisphere. We found that a lesionectomy achieved seizure freedom in 67% of cases in tumor or MCD but in only 20% of other etiologies, suggesting that attempting a “smaller surgery” to avoid injuring eloquent cortex may be appropriate in selected cases of tumor/MCD but is rather ill-advised with other “unfavorable etiologies” (12). Invasive EEG recordings with subdural grids or depths or the use of stereo–EEG are more extensively used for better delineation of the epileptogenic zone and for extraoperative functional mapping optimizing resections with multiple reports showing very promising seizure outcome data. Caicoya et al. (101) found that five of seven occipital lobe epilepsy patients who underwent tailored resections guided by subdural EEG data were seizure free after a mean follow-up of 24.3 months. Cukiert et al. (102) reported on 16 patients with intractable extratemporal epilepsy who had either normal or “nonlocalizing” MRI, finding that 13/14 were rendered seizure free with resections that used subdural EEG information. The use of preoperative invasive monitoring has even been shown in one report to actually correlate with a more favorable outcome in a large cohort of extratemporal resections, consisting mostly of posterior cortex surgeries (83).
PSYCHIATRIC OUTCOMES AFTER EPILEPSY SURGERY
Epilepsy surgery, especially when successful, appears to reduce the prevalence of commonly observed psychiatric comorbidities of epilepsy, including depression and anxiety. Kanner et al. (103) reported a total remission rate off psychotropic medication in 45% of patients who underwent epilepsy surgery. The impact on psychotic disorders, however, is less clearly defined: It varied from unchanged in most cases to improved psychotic status/and or level of functioning (103). Conversely, patients may undergo an exacerbation of an underlying psychopathology or develop de novo psychopathology after surgery. In a study by Wrench et al. comparing the psychiatric outcomes following temporal versus extratemporal resections over a 3-month period, it was found that although both groups had similar baseline rates of depression and anxiety, and more patients were seizure free after a temporal than after an extratemporal resection, the psychiatric outcome was significantly worse in the temporal resections group: At 1 month after surgery, 66% of TL versus 19% of ETL patients reported symptoms of anxiety or depression, which persisted until the 3-month follow-up in 30% of TL and 17% of ETL. In addition, by the 3-month follow-up, 13% of ATL patients had developed a de novo depression as opposed to none in the ETL group. More notably, the occurrence of any of those psychiatric comorbidities was not related to seizure freedom (104). This reinforces the need to carefully evaluate and consider psychiatric outcomes after epilepsy surgery as an independent—albeit intimately connected—entity to the seizure outcomes.
PSYCHOSOCIAL OUTCOMES AFTER EPILEPSY SURGERY
The goals of surgery, as identified by epilepsy patients, extend beyond seizure control, to include driving, regaining or improving employment, and overall independence (104). Intimately linked to these goals is the absence of any “functional” worsening due to surgery, as might occur with a new neurologic deficit, memory loss, or language disturbance. A “successful” surgery is one where seizures are controlled and where the patients’ psychosocial goals materialize into an improved QOL. Several studies have found that for optimal improvement in QOL measurements, complete seizure freedom (even from auras) is required (41,105). Other possible predictors of an improved QOL include a higher presurgical IQ score, younger age at surgery, and a more stable mood at baseline (105). Studies evaluating the psychosocial and educational impacts of surgery in the pediatric population are very limited, but do suggest meaningful improvements in educational attainments and later employment (105).
SURGICAL COMPLICATIONS AFTER FOCAL EPILEPSY SURGERY
The main goal of the pre- and intraoperative evaluation for epilepsy surgery is to identify possible candidates in whom surgical intervention will totally or partially control seizures without increasing neurologic deficits or general morbidity.
In general, we can divide complications in focal neocortical epilepsy surgery based on pathophysiologic mechanisms into
Surgical Complications:
- Infection
- Hematoma
- Brain swelling
- Hydrocephalus
- Vascular compromise (arterial or venous)
Injury to Eloquent Areas of the Brain Causing Neurologic Impairment:
- Hemiparesis
- Hemiplegia
- Visual field defect
- Aphasia
- Alexia
- Neuropsychological impairment (deficits in cognition, memory, language, attention, and concentration)
Psychosocial Impairment:
- Family and interpersonal relationships
- Self-esteem
- Vocational/educational
Psychiatric Impairment:
- Depression
- Anxiety
- Psychosis
In regard to surgical procedures related to neocortical focal epilepsy, we can classify complications due to focal neocortical resections as follows:
Diagnostic Procedures:
- Complications associated with subdural grid and strip electrodes, depth electrode, and SEEG
Therapeutic Procedures—Resective Surgery:
- Complications associated with frontal (mesial and lateral) resections
- Complications associated with temporal lobe resections
- Complications associated with parietal and occipital resections
Diagnostic Procedures
Subdural Grids/Strip Electrodes, Depth Electrode, and SEEG Complications
When noninvasive studies remain nonconcordant or inconclusive regarding the localization and the extent of the seizure- onset zone and/or the eloquent cortex, invasive studies using subdural grids, strips, or depth electrode may be needed (106). Jayakar et al. proposed the following relative indications for the evaluation with invasive monitoring: Normal structural imaging, extratemporal location, divergent noninvasive data, and encroachment on eloquent cortex, tuberous sclerosis, and cortical dysplasia (106). Rosenow and Lüders (107) recommended the use of invasive monitoring only in patients with focal epilepsy (single focus) in whom there is a clear hypothesis regarding the location of the epileptogenic zone (derived from noninvasive studies).
The intracranial placement of subdural grid electrodes via craniotomy has received increasing acceptance over the past decade. Invasive EEG monitoring by subdural grid electrodes facilitates prolonged electrographic assessment as well as extraoperative functional brain mapping of the superficial cortex. Also, it is particularly important in pediatric cases in which awake surgery and intraoperative functional mapping are often difficult.
The principal complications of grid electrode implantation include infection and subdural hematoma formation, which may be associated with neurologic deficits, elevations of intracranial pressure (ICP), and even death (108–110). Other complications may include brain swelling and arterial or venous infarctions (Fig. 90.4). In a recent series, of the 228 cases from 9 centers, the reported complications included infection, hemorrhage with transient deficit, increased preexisting hemiparesis, aseptic necrosis of the bone flap, and transient elevations in ICP (111). In an individual series from the Cleveland Clinic, an initial infection rate of 22% declined to 7% when subcutaneous tunneling of electrode cables was instituted (112). More recently, routine use of perioperative antibiotics and watertight dural closure with sutures at cable exit sites has been advocated in our group (Awad, personal communication, 1992). Since these modifications were introduced, the infection rate has declined markedly.
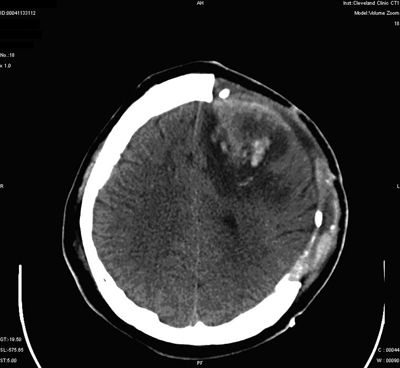
Figure 90.4. Complication of subdural grid placement: Venous infarction located in the left frontal lobe region after subdural grid placement. Postoperative CT after subdural grid removal and bone decompression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








