Fig. 1
Entry site preference of pseudotyped lentiviral vectors into neuronal cells. Lentiviral vector pseudotyped with VSV-G mainly enters the cell body areas of neurons, whereas the HiRet/NeuRet vectors with FuG-B/B2 or FuG-C enter nerve terminals and are transported within axons through retrograde transport to the cell body. Viral RNA is reverse-transcribed and the DNA intermediates are transported into the nucleus and integrated into the host genome, resulting in the expression of the transgene
2 Gene Transduction Property of Lentiviral Vector Pseudotypes
2.1 VSV-G-Pseudotyped Vectors
The pattern of gene transduction in the nervous system by VSV-G-pseudotyped HIV-1 lentiviral vector has been characterized in some animal species. Injection of the VSV-G-pseudotyped vector into the striatum in mice transduces both neuronal and glial cells around the injection site [43]. The VSV-G pseudotype preferentially transduces neuronal cells over glial cells in the mouse striatum, and the transduction efficiency into neurons and glia, defined as the percentage of the number of transduced neurons or glia divided by the total number of transduced cells, is 90 % and 7 %, respectively [36]. The preferential gene transduction of the VSV-G pseudotype into neuronal cells is observed in the brain stem of rats (the transduction efficiency into neurons and glia: 56 % and 26 %, respectively) [44] and in the striatum of rats (the efficiency into neurons and glia: 68 % and 22 %, respectively) and monkeys (the efficiency into neurons and glia: 51 % and 38 %, respectively) [45]. In addition, the VSV-G vector efficiently introduces the transgene into neural stem/progenitor cells when injected into the dentate gyrus and the subventricular zone [46–49].
A few studies report gene transfer through retrograde transport by the HIV-1-based vector pseudotyped with VSV-G into a small number of neurons in the substantia nigra pars compacta (SNc) after an intrastriatal injection [22, 45]. In contrast, retrograde gene transfer has not been observed in most experiments with the VSV-G pseudotype [24, 36, 43]. These observations suggest that the entry of VSV-G-pseudotyped vector may be mediated principally through the interaction with neuronal cell bodies, although the vector entry may partially involve the interaction with the dendritic structures. VSV-G appears to interact with phosphatidylserine, phosphatidylinositol, and gangliosides [50–52], although a recent study excludes the possibility that phosphatidylserine is the receptor for VSV-G [53]. Another recent study demonstrates that the endoplasmic reticulum chaperone gp96 is essential for infection of VSV and that cells without gp96, or with catalytically inactive gp96, do not bind VSV-G-bearing virions [54]. gp96 seems to be responsible for the presentation of functional VSV-G receptors on the cell surface. Identification of the VSV-G receptor will facilitate the understanding of molecular mechanisms that explain the localized entry of VSV-G-pseudotyped vector.
2.2 HiRet/NeuRet Vectors
The HiRet vector is a pseudotype of the HIV-1-based vector containing the fusion glycoprotein type B (FuG-B), which consists of the extracellular and transmembrane domains of RV-G (derived from the challenge virus standard strain) and the cytoplasmic domain of VSV-G (see Fig. 2) [38]. Injection of the HiRet vector into the dorsal striatum of mice produces efficient retrograde gene transfer into neuronal populations that innervate the striatum, such as the cerebral cortical areas, intralaminar thalamic nuclei, and ventral midbrain (SNc), showing a 8- to 14-fold greater efficiency in each brain region than that obtained after injection of the RV-G pseudotyped vector. Injection of the HiRet vector into the ventral striatum generates retrograde gene transfer into the piriform cortex, subiculum, basolateral nucleus of the amygdala, and lateral hypothalamus; and the vector injection into the medial prefrontal cortex leads to gene transduction of the cingulated cortex, hippocampus, and mediodorsal and ventromedial thalamic nuclei. Around the injection site in the dorsal striatum, the HiRet vector transduces a large number of astroglial cells (~75 %) and a much smaller number of neurons (~20 %), and this transduction pattern around the injection site is similar to that of the RV-G-pseudotyped vector. In addition, the RV-G sequence in FuG-B can be replaced with the sequence derived from another rabies virus strain: Pasteur virus (termed FuG-B2); a HiRet vector pseudotyped with FuG-B2 produces a greater level of retrograde gene transfer, showing an approximately 1.3-fold increase than the original FuG-B-pseudotyped vector [39].
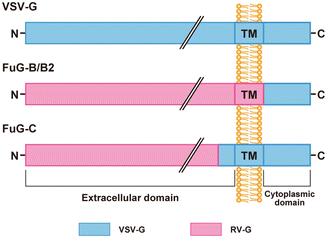

Fig. 2
Structure of viral envelope glycoproteins. The extracellular, transmembrane (TM), and cytoplasmic domains are shown. FuG-B/B2 consist of the extracellular and transmembrane domains of RV-G fused to the cytoplasmic domain of VSV-G . The amino acid sequences of RV-G segments in FuG-B and FuG-B2 are those of glycoproteins derived from the challenge virus standard and Pasteur virus strains, respectively. FuG-C contains the N-terminal region of the extracellular domain of RV-G and the membrane-proximal region and the transmembrane/cytoplasmic domains of VSV-G
The NeuRet vector is another pseudotype of the HIV-1-based vector showing high efficiency of retrograde gene transfer with fusion glycoprotein type C (FuG-C), which is composed of the N-terminal region of the extracellular domain (439 amino acids) of RV-G, and a short C-terminal region of the extracellular domain or membrane-proximal region (16 amino acids), and the transmembrane/cytoplasmic domains of VSV-G (see Fig. 2) [40]. Intrastriatal injection of the NeuRet vector results in efficient retrograde gene transfer into neuronal populations innervating the striatum in a similar fashion to the gene transfer by the HiRet vector. However, the efficiency of retrograde gene delivery into different neural pathways varies between these two vectors; for instance, the gene transfer into the corticostriatal pathways is relatively higher in the NeuRet vector than in the HiRet vector, whereas the transfer into the thalamostriatal pathway is the opposite [55]. More interestingly, the NeuRet vector transduces only a small number of neurons (~6 %) around the injections site, but the transduction of glial cells is less efficient (~0.3 %). Gene transfer into neural stem/progenitor cells by the NeuRet vector also shows a low level compared with VSV-G- or RV-G-pseudotyped vector [40]. Thus, the NeuRet vector shows cell-type specificity for gene transfer and it does not transduce dividing cells in the nervous system, including glial and neural stem/progenitor cells. One significant issue on the therapeutic use of lentiviral vectors is transgene integration into the gene loci adjacent to cellular oncogenes that lead to tumorigenesis [56–58]. The property of NeuRet vector that suppresses gene transduction into dividing cells will be beneficial to reduce the risk of tumorigenesis and improve the safety of future gene therapy trials.
The use of different combinations of RV-G and VSV-G segments for pseudotyping affects the extent of retrograde gene transfer and the cell type specificity of gene transduction around the injection site. Pseudotyping with FuG-B/B2, as compared with RV-G, increases the transduction into cultured cells, whereas it does not appear to influence the yield of vector particles. This pseudotyping results in enhanced retrograde gene transfer into various neural pathways. Substitution of the cytoplasmic domain of RV-G with the corresponding part of VSV-G may change the interaction with host cells or the transduction level of the pseudotyped vector. Pseudotyping with FuG-C, in addition to its enhanced retrograde gene transfer, suppresses gene transduction into dividing cells around the injection site. These results suggest that the N-terminal region of the RV-G extracellular domain of 439 amino acids is responsible for retrograde gene transfer, probably by promoting the entry into axonal terminals of neuronal cells. Amino acid residues involved in rabies virus virulence are reported to be localized in this extracellular domain [59, 60]. In addition, the results of FuG-C pseudotyping suggest that the membrane-proximal region of the extracellular domain may be implicated in the determination of the cell-type specificity of gene transduction around the injection site.
The entry of the pseudotyped vectors into nerve terminals may be mediated via the receptor for RV-G. Previous studies suggest that RV-G interacts with highly sialylated gangliosides [61] and certain receptor proteins, including the nicotinic acetylcholine receptor (nAChR) α-subunit in the neuromuscular junction [62, 63], the low-affinity nerve growth factor receptor or p75NTR [64], and the neural cell adhesion molecule [65]. However, nAChR is located at the postsynaptic muscle membrane, but not at the presynaptic terminals, suggesting the presence of other receptor molecules that mediate viral entry into neuronal cells [66]. Furthermore, there is evidence that p75NTR is not necessary for rabies virus infection [67]. Identification of the RV-G receptor will promote the understanding of mechanisms that mediate the entry of the HiRet/NeuRet vectors into the nerve terminals.
3 Delivery of Transgene into Target Neurons Through Retrograde Gene Transfer
3.1 Gene Transfer into Nigrostriatal Dopamine Neurons
Parkinson’s disease is the most common motor system disorder resulting from selective degeneration of nigrostriatal dopamine neurons. The HIV-1 vector pseudotyped with VSV-G has been used for gene therapy trials in rodent and nonhuman primate models to deliver transgenes required for survival and protection of dopamine neurons such as glial cell line-derived neurotrophic factor, neurturin, and parkin into the striatum or the SNc [4–10]. In this review, we describe a model experiment to evaluate the capability of the NeuRet vector for gene transfer via retrograde transport into the nigrostriatal dopamine system in nonhuman primates [40]. Injection of the NeuRet vector carrying the gene for green fluorescent protein (GFP) into the striatum (putamen) of crab-eating monkeys results in the occurrence of a large number of GFP-positive cells in the SNc, and transgene expression is observed in the majority of SNc dopamine neurons (~70 %) (Fig. 3). Thus, the NeuRet vector system makes it possible to efficiently introduce transgenes involved in neuronal survival and protection for Parkinson’s disease therapy through retrograde gene transfer after intrastriatal injection of the vector.
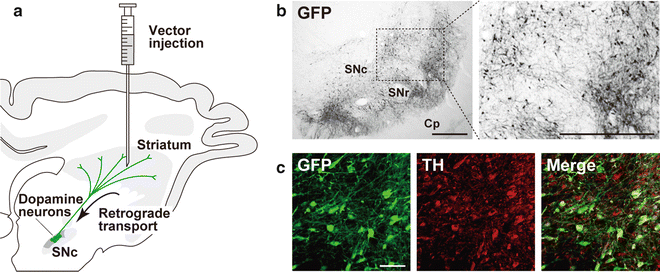

Fig. 3
Retrograde gene transfer into nigrostriatal dopamine neurons after intrastriatal injection. (a) Schematic illustration of intrastriatal injection of a lentiviral vector for retrograde gene transfer in the macaque monkeys. The NeuRet vector encoding the GFP transgene (1.2 × 1010 copies/ml, 5.0 μl × 10 sites) was injected into the striatum (putamen) of the crab-eating monkeys. (b) GFP immunohistochemistry. After 4 weeks, sections through the SNc were prepared, and stained by GFP immunohistochemistry. A number of GFP-positive neurons are visualized in the SNc of the injected monkeys. Cp cerebral peduncle, SNr substantia nigra pars reticulata. (c) Double immunohistochemistry for GFP and tyrosine hydroxylase (TH). The majority of GFP-positive neurons exhibited TH immunoreactivity, indicating efficient retrograde gene transfer into the nigrostriatal dopamine neurons. Scale bars: 500 μm (b) and 50 μm (c). (The data are modified from ref. [40])
3.2 Gene Delivery into Motor Neurons
Motor neuron diseases, including amyotrophic lateral sclerosis and spinal muscular atrophy, are characterized by progressive muscle weakness and paralysis resulting from degeneration of motor neurons in the spinal cord and brain . Intramuscular injection of an adenoviral vector encoding genes required for neuronal survival and protection, such as brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, and neuronal apoptosis inhibitory protein, prevents motor neuron death in axotomy-induced injury models [27–31]. Adeno-associated virus serotype 6 and 9 vectors also deliver desired transgenes into motor neurons following an intramuscular injection [68–70]. Recently, we reported the gene transfer capability of the HiRet vector into motor neurons in rodents via retrograde transport [71]. Injection of the HiRet vector (pseudotyped with FuG-B2) encoding GFP into the hindlimb muscles in mice leads to the transduction of motor neurons in the spinal cord at the lumbar level (Fig. 4). The gene transfer efficiency of the HiRet vector is much higher than that of the RV-G-pseudotyped vector, showing a 4.6-fold increase. Injection of the HiRet vector into the tongue muscle also produces a large number of GFP-positive cells in the hypoglossal nucleus, showing a 14.8-fold increase in efficiency compared with the RV-G vector. The efficiency of retrograde gene transfer of the NeuRet vector into motor neurons is not comparable to that of the HiRet vector. Therefore, the HiRet vector system provides a useful approach for efficient introduction of the genes involved in the survival of motor neurons through retrograde gene transfer.
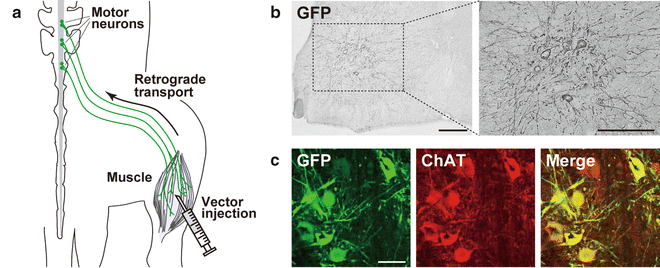

Fig. 4
Retrograde gene transfer into spinal cord motor neurons after intramuscular injection. (a) Schematic illustration of intramuscular injection of a lentiviral vector for retrograde gene transfer in mice. The HiRet vector (FuG-B2 pseudotype) encoding the GFP transgene (5.0 × 1011 copies/ml, 5.0 μl × 6 sites) was injected into the hindlimb muscle of mice. (b) GFP immunohistochemistry. After 4 weeks, sections through the lumbar spinal cords were prepared, and stained by GFP immunohistochemistry. GFP-positive neurons are seen in the ventral horn of the spinal cord. (c) Double immunohistochemistry for GFP and choline acetyltransferase (ChAT). GFP-positive neurons express ChAT showing the retrograde gene transfer into spinal cord motor neurons. Scale bars: 200 μm (b) and 50 μm (c). (The data are modified from ref. [71])
4 Concluding Remarks
Pseudotyping with different viral envelope glycoproteins not only confers neurotropism to lentiviral vectors, but also converts the preference of vector entry site s into neuronal cells. The pseudotyped HIV-1 vectors with neurotropism are distinguishable based on the vector entry site preference: One group is the VSV-G -pseudotyped vector that preferentially enters cell body areas (somata/dendrites) of neurons and transduces them, and another group contains the HiRet/NeuRet vectors pseudotyped with FuG-B/B2 or FuG-C that predominantly enter axon terminals of neurons and are transported retrogradely into their cell bodies, displaying enhanced retrograde gene delivery . In addition to the general use of the VSV-G-pseudotyped vector, the application of our NeuRet vector results in gene transfer into a large number of nigrostriatal dopamine neurons that are the major target for gene therapy of Parkinson’s disease. Furthermore, intramuscular injection of the HiRet vector achieves gene transfer into motor neurons in the brain and spinal cord that are the target for gene therapy of motor neuron diseases. Recently, the efficiency of retrograde gene transfer by the NeuRet vector was further improved by optimizing the junction of RV-G and VSV-G segments in the membrane-proximal region of fusion envelope glycoproteins [72]. A better understanding of the molecular mechanisms underlying vector entry into neuronal cells will contribute to the continuous development of genetic therapeutic approaches for the treatment of intractable neural diseases.
References
1.
2.
3.
4.
5.
Palfi S, Leventhal L, Chu Y et al (2002) Lentivirally delivered glial cell line-derived neurotrophic factor increases the number of striatal dopaminergic neurons in primate models of nigrostriatal degeneration. J Neurosci 22:4942–4954PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






