Fig. 1
Schematic summary showing fiber projections of orexin neurons (top) and afferent pathways to orexin neurons (bottom) in the rodent brain. Nomenclatures: see the list of abbreviations
1.1 Projection within the Hypothalamus
Orexin neurons innervate almost all regions of the hypothalamus. Dense fiber projections with varicose terminal structure were found widely in the LH, the dorsomedial hypothalamic nucleus (DMH), the supramammillary nucleus (SuM), the tuberomammillary nucleus (TMN), the posterior hypothalamic area (PH) and the arcuate nucleus (Arc). Relatively dense fibers were also found in the anterior hypothalamic area (AHA), the paraventricular nucleus (PVH), the ventromedial hypothalamic nucleus (VMH) and the preoptic area (POA), although no clear innervations were found in the suprachiasmatic nucleus (SCN). Both OX1 and OX2 receptors were widely expressed in the hypothalamus, but some nuclei showed uneven expression of the receptors; OX2 receptor is dominant in the Arc, LH, PVH, SuM and TMN, in contrast, OX1 receptor is dominant in VMH. These findings strongly suggest that orexin is involved in many hypothalamic functions, such as sleep/wake control, thermoregulation, feeding/drinking, neuroendocrine control and autonomic functions.
1.2 Projection to the Thalamus
A moderate density of orexin fibers were found along the midline regions of the thalamus, including the paraventricular nucleus (PVT), the central medial nucleus (CM), and the lateral habenula. Strong signals of OX1 and OX2 receptors were found in the PVT, and moderate signals of OX2 receptors were found in other medial thalamic regions including the CM. Though these thalamic nuclei are considered to function as a relay of sensory stimuli to the cortex and activate cortical arousal (Anderson et al. 2005; Fuller et al. 2011), the physiological importance of orexin signaling in the thalamus needs to be examined.
1.3 Projection to the Forebrain
Relatively dense fibers were found in the substantia innominata (SI), the bed nucleus of the stria terminalis (BST), the lateral and medial septal nuclei (LS, MS), and the diagonal band of Broca (VDB, HDB). A moderate density of the fibers was found in the anterior area and the central nucleus of the amygdala (AAD, CeM). A low density of the orexin fibers was widespread in the cerebral cortex and the hippocampus. Relatively strong expressions of OX2 receptors were found in the MS and VDB. In the hippocampus, a moderate expression of OX2 receptors were seen in the CA2-3 fields, though the fiber density was low. In the BST, both OX1 and OX2 receptors were expressed moderately. These results suggest that orexin plays important roles in the basal forebrain arousal mechanism, the septohippocampal functions and limbic systems (Arrigoni et al. 2010; Zhang et al. 2006). Interestingly, a strong expression of OX1 receptor was found in the tenia tecta with a moderate density of the fiber projection toward the prefrontal cortex (insular, infralimbic and prelimbic cortex) and the anterior olfactory nuclei, though the physiological importance of these pathways has not been examined yet.
1.4 Projection to Midbrain and Pons
Relatively dense fibers were found in the periaqueductal gray (PAG), the dorsal and median raphe nuclei (DR, MnR), the pedunculopontine tegmental nucleus (PPTg), the ventral tegmental area (VTA) and the substantia nigra compact/lateral part (SNC, SNL). The PAG, DR and PPTg express both OX1 and OX2 receptors, and are recognized as a component of sleep/wake control mechanism. The VTA and SNC also express both OX1 and OX2 receptors, and are associated with the motivation/reward, addiction and motor control mechanisms. Interestingly, a high level of OX2 receptor mRNA expression was seen in the pontine nuclei, however, the fiber projection was low level and the physiological importance of orexin in the pontine nuclei is unknown.
1.5 Projections to the Brainstem
A very dense fiber projection was found in the locus coeruleus (LC, A6). Other noradrenergic cell groups (A4, A5 and A7) were also innervated moderately, and these noradrenergic neurons dominantly express OX1 receptors. Moderately dense fibers were found in the parabrachial nucleus (PB) and the laterodorsal tegmental nucleus (LDTg) with the low level expressions of both OX1 and OX2 receptors. The LC, PB and LDTg are closely related to the control of sleep/wake behavior. Moderate dense fibers were also found in the nucleus of the solitary tract (Sol) and the dorsal motor nucleus of vagus, and a scattered fiber projection was found in broad areas of the reticular formation. These areas express both OX1 and OX2 receptors diffusely, and orexin may affect the autonomic functions through these nuclei.
2 Afferents to Orexin Neurons
The source of afferents to the orexin neurons have been characterized in detail using a combined retrograde (cholera toxin B, CTB) and anterograde (biotinylated dextrans, BD) tracing technique in the rat brain (Yoshida et al. 2006). This, when combined with the results of Sakurai, who generated a new line of transgenic mice expressing a retrograde tracing fusion protein (C-terminal fragment of tetanus toxin and green fluorescent protein, TTC::GFP) selectively in orexin neurons (Sakurai et al. 2005), provides conclusive results of the afferents in the rodent brain.
2.1 Hypothalamic Inputs to the Orexin Neurons
The Hypothalamus provides the heaviest innervation of the orexin neurons, with the densest inputs originating in the POA and PH. Most of hypothalamic regions innervate the medial part of the orexin field. Inputs to orexin neurons from the hypothalamic area are:
Medial preoptic area. The medial preoptic area (MPA) heavily innervates the orexin neurons. The lateral part and caudal part of MPA are preferentially and heavily innervated neurons in the medial and perifornical parts of the orexin field, especially just dorsomedial to the fornix. Fibers from the MPA mainly follow the mediodorsal path into the orexin field.
Lateral preoptic area. The orexin neurons receive heavy inputs from the center of the ventrolateral preoptic area (VLPO). The inputs are most abundant dorsal and medial to the fornix. Although the input patterns to the orexin neurons are similar, fewer appositions are seen from the lateral preoptic area (LPO, lateral and dorsal to the VLPO). Fibers from the LPO course caudally through the medial forebrain bundle (mfb), but the fibers from VLPO take the mediodorsal path.
Anterior hypothalamic area. Moderate inputs to the perifornical area of the orexin field are seen from the anterior hypothalamic area (AHA), however, neurons of the suprachiasmatic nucleus (SCN) innervate the orexin neurons very sparsely in the rat brain and no GFP-positive cells was found in the SCN of orexin/TTC::GFP transgenic mice.
Ventromedial and dorsomedial hypothalamus. The VMH neurons project moderately into the orexin neurons. Most of inputs are found medial to the fornix. The fibers from the VMH extend dorsally up into the medial part of the orexin field, DMH, and then into the dorsal hypothalamic area. The DMH neurons also show direct input to orexin neurons in orexin/TTC::GFP transgenic mice.
Posterior hypothalamus. The neurons in the PH heavily innervate orexin neurons, mostly dorsal and medial to the fornix. These fibers from the PH enter the dorsomedial part of the orexin field and then course in a ventrolateral direction. Interestingly, the TMN neurons show no direct input to orexin neurons in orexin/TTC::GFP transgenic mice.
Supramammillary nucleus. The SuM targeted the orexin neurons lightly. Fibers from the SuM innervate the perifornical and medial parts of the orexin field and also extend into the DMH, VMH, and Arc.
These afferents strongly suggest that orexin neurons are associated with many hypothalamic functions. In addition to above inputs, orexin neurons receive local excitatory input between themselves and/or from glutamatergic interneurons (Li et al. 2002; Sakurai et al. 2005; Yamanaka et al. 2010). This self-conditioning mechanism may help orexin neurons remain active for a long time when necessary, for example, to arouse animals during a variety of active behavior (España et al. 2003; Mochizuki et al. 2004, 2006; Alexandre et al. 2013). In contrast, the heaviest, presumably inhibitory input originates in the VLPO and MPA; possible sleep-active neurons that produce GABA and galanin (Sherin et al. 1998; McGinty et al. 2004). The VLPO mainly projects to the medial and perifornical parts of the orexin field, and orexin neurons in these regions show the greatest reduction in the activity during sleep (Estabrooke et al. 2001). Regions that control metabolism and feeding such as the Arc, DMH and VMH are likely to modulate the orexin neurons.
Sleep/wake behavior is closely regulated by the circadian biological rhythm, however, orexin neurons rarely receive the direct projection from the SCN, the main circadian oscillator in the mammalian brain (Reppert and Weaver 2002). An alternative multi-synaptic pathway was suggested by the neuroanatomical tracing and behavioral studies that the output of the SCN was first mediated by the neurons in the subparaventricular zone (SPZ), then to the DMH and finally to orexin neurons (Lu et al. 2001; Chou et al. 2003; Saper et al. 2005, 2010).
2.2 Forebrain Afferents to the Orexin Neurons
Most inputs from the forebrain regions innervate orexin neurons diffusely across the entire field, with slightly more projections in the perifornical region. Inputs to orexin neurons from the forebrain and limbic areas are:
Prefrontal cortex. Although the infralimbic cortex (IL) projects dense fibers to the LH, these neurons innervate orexin neuron relatively lightly. Neurons of the cingular cortex project fibers to the zona incerta and subincertal nucleus, but rarely make direct contact with orexin neurons.
Septal nuclei. The LS is one of the largest inputs to the orexin neurons, topographically innervating different parts of the orexin field. The intermediate part of the LS mainly targets the medial part of the field and the dorsal part of the LS projects to the lateral part. In all areas, the fibers course through the mfb and then radiated throughout the orexin field. In orexin/TTC::GFP transgenic mice, the retrogradely labeled neurons extended to the medial septal nucleus and the diagonal band of Broca, however, these area were largely negative by CTB/BD tracing in the rat brain.
Nucleus accumbens. The rostral and caudal part of the shell of the nucleus accumbens (AcbSh) targets orexin neurons lightly. Fibers from the AcbSh run through the lateral part of the mfb and extended into the lateral part of the orexin field.
Bed nucleus of stria terminalis. The BST is a major source of inputs to the orexin neurons. The ventral part of the lateral subdivision innervates orexin neurons, scattered across the field. The rostral, medial BST lightly innervates the orexin neurons. The posterior part of the medial division moderately innervates the orexin neurons, but the caudal edge of the medial division innervates fewer cells. These fibers pass through the medial part of the mfb to contact orexin neurons across the field, especially in the perifornical region.
Amygdala. The lateral subdivision of the CeM innervates the orexin field, especially to the lateral half of the field. Fibers from the CeM course around the internal capsule to heavily innervate the perifornical region. The anterodorsal and posterodorsal parts of the medial amygdala innervate orexin neurons lightly in the lateral part of the field. In orexin/TTC::GFP transgenic mice, many retrogradely labeled neurons were also found in the basomedial nucleus of amygdala.
Subiculum. The fibers from dorsal subiculum are scarce in the orexin field. The ventral subiculum project few orexin neurons, despite dense fibers in the fornix.
These forebrain inputs may drive orexin neurons in response to various emotional, stress and autonomic stimuli. The prefrontal cortex has an important role in emotional control, and suppression of these areas reduced food-elicited cataplexy in orexin knockout mice (Oishi et al. 2013). The amygdala is also one of the well-studied regions for processing emotional stimuli, and large neurotoxic lesion in the CeM and basolateral amygdala reduced emotion/motivation-triggered cataplexy in orexin knockout mice (Burgess et al. 2013). The cholinergic neurons in the basal forebrain, widely spread in the septal nuclei, the diagonal band of Broca, the magnocellular preoptic nucleus (MCPO) and the SI, are supposed to work for wake maintenance, and 20 % of orexin neurons were excited electrophysiologycally with a muscarinic agonist, carbachol (Yamanaka et al. 2003; Sakurai et al. 2005). However, more recent study suggested that the major afferents from the basal forebrain are non-cholinergic innervations (Agostinelli et al. 2013). Other inputs, such as the AcbSh and BST would influence stress, aggression, and anxiety, and all of these forebrain inputs may be important to activate orexin neurons when emotional stimuli drive arousal and autonomic responses in animals (Kayaba et al. 2003).
2.3 Brain Afferents to the Orexin Neurons
Projections from the SNR, DR, and VTA preferentially innervate orexin neurons in the lateral part of the orexin field. Many of these brainstem inputs project primarily to the ipsilateral orexin field, but fibers from the DR, ventrolateral PAG, and LC project bilaterally. Inputs to orexin neurons from brainstems are:
Substantia nigra. Neurons in the reticular part of the substantia nigra (SNR) project to the orexin neurons. Numerous fibers from the SNR course through the LH to orexin neurons in the lateral part of the field, but actual contacts are sparse in the medial and perifornical parts of the orexin field.
Ventral tegmental area. Neurons in the rostral and caudal VTA moderately innervate the orexin neurons with direct contacts, especially in the perifornical and lateral part of the field. In orexin/TTC::GFP transgenic mice, however, the retrogradely labeled neurons were limited to the most caudal part of the VTA and possibly non-tyrosine hydroxylase (TH) positive group.
Periaqueductal gray matter. Neurons in the ventrolateral PAG make 1–2 appositions on orexin neurons, scattering throughout the orexin field. The neurons in the caudal lateral PAG project to the medial part of the orexin field. Both fibers ascend from the mfb into the dorsal hypothalamus.
Raphe nuclei. The central portion of the anterior DR very lightly innervates the orexin neurons, mainly in the lateral orexin field. Fibers ascend bilaterally through the mfb and course through the orexin field. In contrast, in orexin/TTC::GFP transgenic mice, many neurons in the MnR and paramedian raphe nucleus (PMnR) showed retrogradely-labeled signals, suggesting the major source of serotonergic input to orexin neurons instead.
Lateral parabrachial nucleus. The rostral part of lateral parabrachial nucleus (LPB) lightly innervate the orexin neurons bilaterally. The cells with appositions are scattered throughout the orexin field.
Locus coeruleus. The rostral LC projects a few fibers to the orexin field, whereas the caudal LC has no fiber projection in the field. In orexin/TTC::GFP transgenic mice, no retrogradely-labeled neurons was found in the LC, suggesting that LC is not the major source of noradrenergic input to orexin neurons. In contrast, the neurons in the precoeruleus area (PreC) mildly innervate the orexin field, mainly in the perifornical and medial parts.
Compared to the dense fiber projections from orexin neurons to wake-active nuclei in the brain stem (LC, DR, PB), afferent signals from these groups were considered minor. These neurons may work mostly as output pathway of orexin signaling. Many serotonergic neurons around the MnR innervate orexin neurons and may form a feedback circuit to modulate the activity of orexin neurons (Muraki et al. 2004). Orexin neurons in the lateral part of the field are activated when an animal anticipates a reward such as drugs or food (Harris et al. 2005), and these neurons may be more influenced by regions that regulate reward, emotion, and autonomic function such as the VTA, SN, or ascending visceral afferents.
3 Functional Mapping of Orexin Neurotransmission
Recent progress in molecular biology techniques, such as Cre-loxP gene recombination combined with gene-targeting elements, light-gated bacterial ion channels/pumps, drug-specific mutant receptors and transcription activators, enables us to study roles of focal brain regions or specific group of neurons in a variety of physiology and behavior in animals. Using these new genetic techniques, many new lines of transgenic mice, knock-out/in mice and recombinant viral vectors have been developed for detailed neuroanatomical and behavioral studies, including sleep/wake studies. Here, we summarize the recent mouse studies addressing the functional mapping of orexin neurotransmission with regard to the sleep/wake control circuitry (Fig. 2). These studies successfully demonstrate the integrated anatomical-physiological evidences of how orexin neurons regulate sleep/wake behavior in the brain.
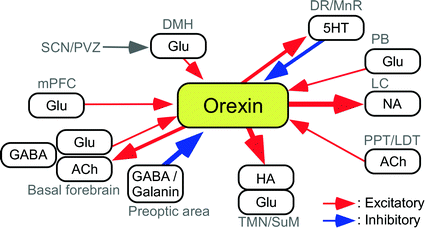

Fig. 2
Circuitry diagram of sleep/wake control by orexin neurons. Orexin neurons activate cholinergic and non-cholinergic neurons in the basal forebrain, histaminergic and other neurons in the TMN/SuM, and noradrenergic neurons in the LC to promote wakefulness. Serotonergic neurons in the DR/MnR may send negative feedback signals to orexin neurons. GABA/galanin neurons in the preoptic area suppress orexin neurons during sleep. Other wake (and REM) active neurons in the brainstem (PB, PPT, LDT) are supposed to modulate the activity of orexin neurons. The circadian clock signal is considered to input from the SCN via the PVZ and the DMH. The mPFC neurons may activate orexin neurons in response to emotional excitation
3.1 Orexin Receptor Rescue in the Posterior Hypothalamus
Loss of orexin signals in the brain causes narcolepsy syndrome in both humans and animals, showing chronic sleepiness, fragmented sleep/wake and cataplexy. One way to map the critical brain regions for orexin neurotransmission is to focally restore the orexin signaling and improve the behavioral deficits in narcoleptic animals. For this purpose, we produced a new line of mouse model in which a loxP-flanked gene-targeting cassette disrupts the transcription of OX2 receptor gene, but normal, eutopic expression of OX2 receptor can be restored by introducing Cre recombinase in the neurons (Mochizuki et al. 2011). These mice were born as OX2 receptor null mice, and had mildly fragmented wakefulness during the active period. We focally injected an adeno-associated viral vector (AAV) coding for Cre recombinase into the posterior hypothalamus, including the TMN (histamine neurons) and the SuM (probably glutamate neurons), and found that the restoration of OX2 in these neurons completely rescued the fragmented wakefulness in these mice. The results indicated that the posterior hypothalamic region works as an important relay pathway of orexin signaling to consolidate wake behavior. However, similar OX2 receptor rescue using OX2-coding viral vector was examined in constitutive OX1/OX2 double receptor knockout mice, showing severe sleep/wake fragmentation than the single receptor mutants, and the induction of OX2 receptor in the posterior hypothalamus was not effective to improve the fragmented wake (Hasegawa et al. 2014). The results suggest that arousal signal from the posterior hypothalamus may need further OX1-mediated downstream relays to fully recover the wake deficit in narcoleptic mice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







