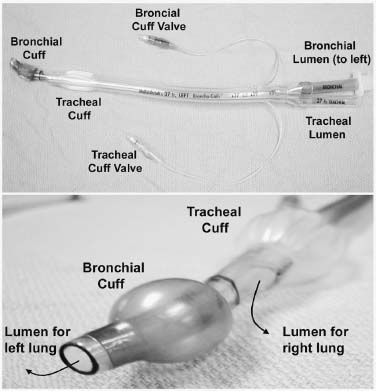
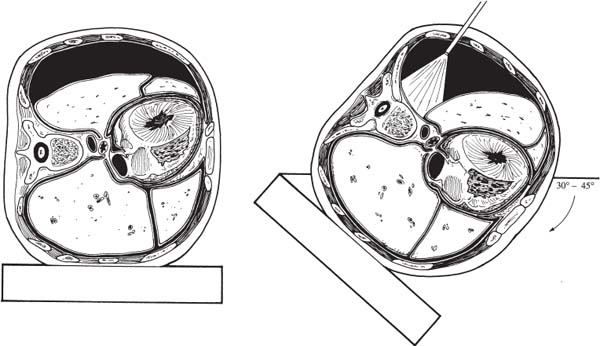
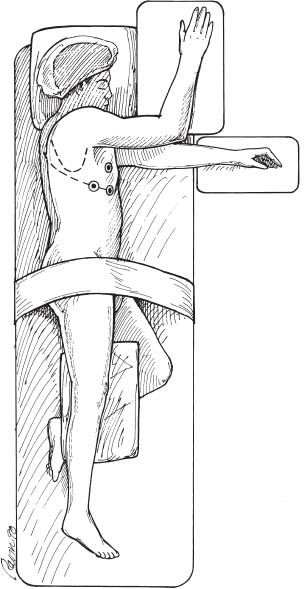
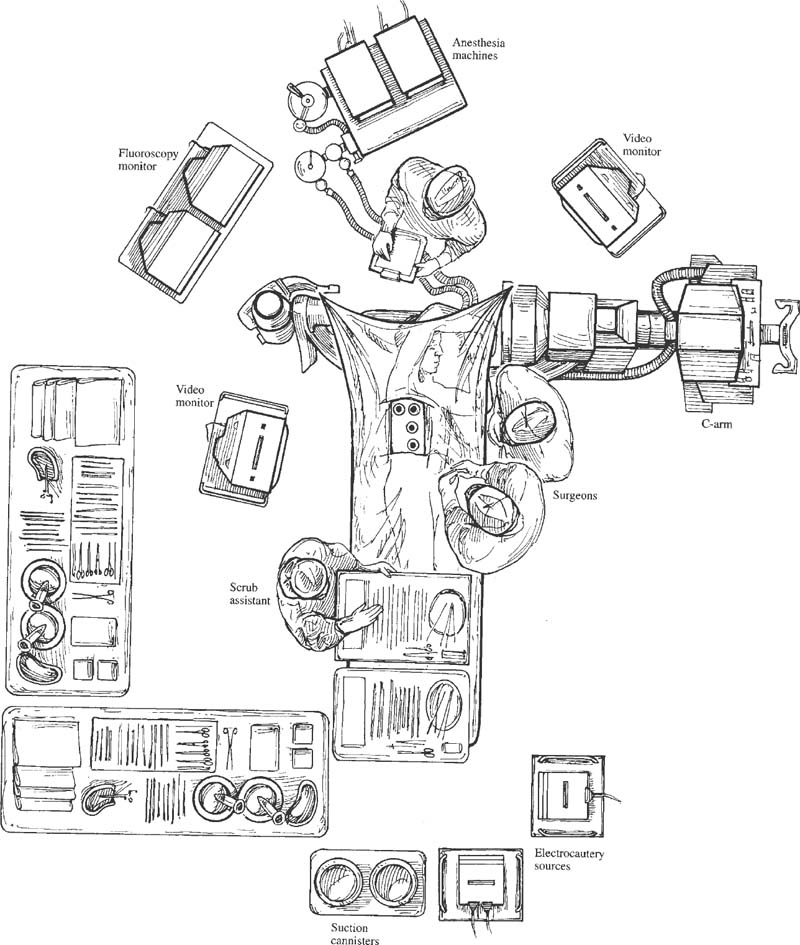
The spinal community has witnessed increasing interest in endoscopic, minimally invasive surgical techniques. Spine surgeons have been encouraged by the prospect of emulating the reduced perioperative morbidity achieved by the general and cardiothoracic surgeons who have championed laparoscopic and thoracoscopic technologies. Endoscopic procedures present anesthetic challenges that are distinct from traditional open procedures. A fairly substantial assembly of specialized equipment is necessary to navigate the spine successfully in a minimally invasive manner. The initial presurgical preparations necessitate careful planning with regard to the operating room setup, patient positioning, and surrounding configuration of all surgical personnel and required equipment. Anesthetic Considerations for Thoracoscopy Although thoracoscopic access to the spine obviates the need for a large thoracotomy incision, it does require retraction or collapse of the nondependent lung for adequate visualization. This and the resultant single-lung ventilation of the dependent side can have significant cardiopulmonary implications, which are important to recognize when undertaking a minimally invasive thoracic procedure. Signs and symptoms of preexisting cardiac and pulmonary disease must be identified and carefully evaluated preoperatively by the appropriate medical consultants. Although the patient may have an adequate baseline level of functioning, he or she will be largely dependent on the pulmonary function of a single lung during a potentially prolonged thoracoscopic procedure. Pulmonary function tests may be warranted in patients with chronic obstructive pulmonary disease (COPD) or other respiratory disorders. The spine patients being managed thoracoscopically for anterior release to correct deformity or for symptomatic thoracic disk herniation fortunately are typically younger and can therefore tolerate single-lung ventilation for the often-prolonged duration of these surgical procedures. Smoking is unfortunately relatively common in patients with spinal disorders. Such patients should be implored to stop smoking at least 6 to 8 weeks prior to surgery because a decrease in sputum production and improvement in ciliary activity will lessen the chance of postoperative respiratory complications.1,2 Of course, beyond the pulmonary implications of smoking that are inherently relevant to the thoracoscopy procedure itself, the adverse effects of smoking on bone fusion and tissue healing are well recognized.3 Perioperative Monitoring Perioperative monitoring of patients undergoing thoracoscopy includes such routine measures as electrocardiogram (ECG), pulse oximetry, blood pressure, temperature, and capnography. Pulse oximetry and capnography provide important information with regard to oxygenation and ventilatory status, and it can provide early warnings for a variety of ventilation problems. Most anesthesiologists place an arterial line for cases involving trespass of the thoracic cavity and single-lung ventilation. Periodic arterial blood gas measurements may also be warranted, especially in patients with preexisting pulmonary disease whose end-tidal CO2 can be misleading. Large-bore peripheral intravenous lines should be established at the onset of the procedure, and consideration should be given to the placement of a central venous catheter, depending on the nature of the procedure. A surgical misadventure leading to a large vessel injury in the thoracic cavity may quickly turn the procedure into a life-threatening hemorrhagic emergency. Central venous access is invaluable for the rapid infusion of resuscitative fluids and pharmacologic agents and for the central monitoring of their effects. A surgical suite setup incapable of rapidly converting to an open thoracotomy and lacking in the necessary blood product resources for urgent hemodynamic resuscitation is suboptimal for minimally invasive thoracoscopic surgery. These deficiencies arguably should be corrected prior to undertaking such procedures. A Swan-Ganz catheter is also useful in settings of large blood loss or in patients with severe preexisting cardiopulmonary disease, although it should be recognized that pulmonary blood-flow alterations associated with single-lung ventilation may invalidate the assumption of uniform pulmonary capillary resistance upon which cardiac output and left-ventricular end-diastolic volume are calculated.4 Single-Lung Ventilation The effects of single-lung ventilation of the dependent lung are arguably the most significant anesthetic consideration for thoracoscopy. Collapse of the nondependent lung is important for visualization of the spine and for minimizing iatrogenic lung injury from various thoracoscopic instruments. Deflation of the nondependent lung is accomplished using a double-lumen endotracheal tube or any one of a variety of specially designed singlelumen tubes with built-in bronchial blockers. In general, complete deflation of the lung takes ~15 to 20 minutes. If collapse of the lung is not sufficient to provide adequate visualization of the thoracic spine, CO2 can be insufflated into the pleural cavity to provide pressure retraction of the mobile lung tissues. Elevating the intrathoracic pressure unfortunately can cause significant hemodynamic alterations, including a tension pneumothorax. It is recommended that insufflation pressures not exceed 10 mm Hg, below which cardiovascular consequences are minimized.5 For the induction of single-lung ventilation, doublelumen tubes are currently favored because of their relative ease of placement, although single-lumen tubes are used most commonly in pediatric patients due to their more compact dimensions. Double-lumen endotracheal tubes have two lumens and two occlusive balloons (Fig. 3–1). The proximal balloon is inflated in the trachea, and the distal balloon is inflated at the entrance to the mainstem bronchus. Many anesthesiologists use a left-sided double-lumen tube regardless of the intended operative side because a right-sided double-lumen tube is more difficult to place without inadvertently occluding the right upper lobe. Single-lung ventilation is then accomplished by ventilating only the dependent lung and opening the nondependent lung to atmospheric pressure, allowing it to collapse naturally. Double-lumen tubes are larger in caliber than are single-lumen tubes; thus, they are more likely to cause tracheobronchial injury. Great care must also be taken to position the two occlusive balloons precisely within the tracheobronchial tree to ensure proper function. Problems with positioning are the most common cause of ventilatory difficulties during single-lung ventilation. Although the presence of breath sounds and ventilation or oxygenation parameters are indicative of accurate tube positioning, direct visualization and confirmation of the tube position are advisable with fiberoptic bronchoscopy.6 FIGURE 3–1 Double lumen endotracheal tube. Note that the tube has two cuffs that can be inflated independently, with the bronchial cuff inflated within the left mainstem bronchus. Such a tube can be used for single-lung ventilation of either right or left lung. For left-lung ventilation, gas exchange occurs through the lumen to the left lung, with the tracheal lumen occluded. For right-lung ventilation, gas exchange occurs through the tracheal lumen, with the left lumen occluded. Several physiologic alterations occur when the nondependent lung is deflated. Ventilation and pulmonary perfusion are normally well matched. This means that areas of lung that receive the most blood also receive the most ventilation. When the nondependent lung is deflated, blood passes through the atelectatic lung, and a ventilation-perfusion (V/Q) mismatch, or shunt, occurs, resulting in suboptimal blood oxygenation. At first, this is compensated by hypoxic pulmonary vasoconstriction (HPV), an autoregulatory mechanism that increases pulmonary vascular resistance in the face of low PaO2 and PvO2. This redirects blood flow to areas of the lung that are well ventilated. HPV ceases to function, however, when ~70% of the lung has collapsed due to a lack of normoxic regions in the lung to which blood can be redirected.7 When full collapse is finally achieved, therefore, significant hypoxia may occur due to both the reduction in pulmonary surface area for gas exchange and the significant V/P mismatch. With the patient in a lateral decubitus position, further deterioration in PaO2 can occur from impaired ventilation within the dependent, “down” lung.8 These physiologic alterations highlight the need to evaluate the cardiopulmonary status of the patient carefully before undertaking a minimally invasive thoracoscopic procedure. Anesthetic Considerations for Laparoscopy Laparoscopic procedures do not directly affect the cardiopulmonary system to the extent of thoracoscopic procedures, but they do have their own systemic influences, which are largely related to the frequent use of intraperitoneal CO2 insufflation and to patient positioning. Pneumoperitoneum with CO2 remains the most common and reliable method of achieving laparoscopic visualization of the lumbar spine. The peritoneal cavity is entered initially with a periumbilical trocar or Veress needle, through which CO2 is introduced to expand the abdomen and to allow for the introduction of other instruments and for exposure to the spine. A peak intraperitoneal pressure no greater than 15 mm Hg is recommended because the hemodynamic consequences at or below this level are relatively modest and well tolerated. In general, the greater the intraperitoneal pressure applied, the greater the compression of the abdominal aorta and inferior vena cava, and hence enhanced potential for hemodynamic disturbance.9 Compression of the aorta increases systemic vascular resistance and afterload, whereas compression of the vena cava decreases venous return and preload. These factors, in association with the hemodynamic effects of anesthetic induction and patient positioning, may transiently reduce cardiac output by as much as 50% of normal.10 These changes tend to normalize within the first 10 to 30 min, but nevertheless represent important considerations in the patient with limited cardiac reserve. Insufflated CO2 itself may have a direct and indirect effect on systemic circulation. The diffusion of CO2 into the systemic circulation results in slightly increased endtidal CO2. The direct effects of this (beyond the mechanical effects of insufflation) are not entirely clear, although it may contribute to acidemia, hypercapnea, and reduced stroke volume,11 particularly in patients with severe pulmonary disease and the impaired ventilatory function to eliminate CO2 (e.g., COPD). Laparoscopy systems that rely on specialized retractor systems to lift abdominal wall contents instead of CO2 insufflation have been described.12 Although this minimizes the potential adverse hemodynamic effects of CO2 insufflation, the advantages of such gasless techniques may be offset somewhat by inferior surgical visualization.13 Endoscopic spine procedures typically require Trendelenburg positioning of the patient to move the abdominal contents superiorly and facilitate access to the L4–L5 and L5–S1 disk spaces. It appears that the establishment of pneumoperitoneum in either the Trendelenburg or reverse Trendelenburg position significantly worsens the hemodynamic abnormalities associated with CO2 insufflation. Both positions affect venous return to the heart, albeit in opposite manners. The reverse Trendelenburg position is associated with significant venous pooling in the lower extremities, leading to decreased preload and a reduction in cardiac output. Trendelenburg positioning results in an opposite physiologic phenomenon (i.e., an increase in venous return to the heart and increased cardiac output).14 In addition to altering hemodynamic function, intraperitoneal insufflation and the Trendelenburg position both push the diaphragm superiorly, thus reducing diaphragmatic excursion, functional residual capacity, and pulmonary compliance. Although these changes may be well tolerated by the young, healthy individual, they may have significant adverse effects on patients with preexisting pulmonary disease. Trendelenburg positioning may also cause the carina to move superiorly and alter the relative position of the endotracheal tube. Sudden intraoperative changes in ventilatory status of the patient during such procedures therefore warrant an immediate evaluation of endotracheal tube position. Although both thoracoscopy and laparoscopy involve several anesthetic considerations that warrant cautious attention, one should not overlook the potential for these minimally invasive procedures to reduce overall morbidity and improve patient care in comparison to open procedures that necessitate larger incisions. The increasing interest in endoscopic surgery as an alternative to large-incision open exposures is a testimony to this. Nevertheless, unique anesthetic issues exist for thoracoscopic and laparoscopic surgery, which need to be acknowledged, particularly in patients with preexisting medical conditions. Operating Room Setup for Endoscopic Spine Surgery Endoscopic spinal procedures are quite resource-intensive in terms of operating room personnel and equipment needs. In addition to the obligatory surgical and anesthetic teams (including assistants and nurses), these procedures may require two or more circulating nurses, a neuromonitoring team, and a dedicated x-ray technician. A thoracic, cardiovascular, or general surgeon proficient in endoscopy is often beneficial as a cosurgeon or assistant to the spine surgeon to assist in the exposure and to provide urgent open access should the need arise. For example, it is estimated that conversion from thoracoscopy to open thoracotomy occurs in 6 to 20% of cases, most often because of the need to gain better access to the surgical pathology.15 A physically large operating room is helpful to accommodate the required personnel and equipment necessary for such minimally invasive procedures as a fluoroscopy machine, multiple video monitors (these need to be positioned on either side of the patient in a direct line of sight for the surgeon and assistant) , and, possibly, image guidance systems. The myriad of specialized endoscopic spinal instruments requires a well-familiarized surgical team of assistants and nurses, a substantial amount of sterile table space, and a cogent strategy to maintain all of the equipment in an orderly fashion within the operative field. The scrub nurse should have the Mayo stand arranged toward the foot of the bed in such a way that such commonly used equipment as the electrocautery and suction are easily delivered to surgeons on either side of the table. FIGURE 3–2 Tilting the patient forward during thoracoscopic procedures will use gravity to move the lung away from the spine and thus facilitate visualization. (With permission from Rosenthal DJ, Dickman CA. Operating room setup and patient positioning. In: Dickman C, Rosenthal DJ, Perin NI, eds. Thoracoscopic Spine Surgery. New York: Thieme Medical Publishers; 1999:104.) In thoracoscopy procedures, the patient is positioned in the lateral decubitus position with the lung and side of the spine to be accessed lying superiorly. Ensuring an exact lateral position is helpful for orientation both visually with the thoracoscope and radiographically with fluoroscopy. A radiolucent table is helpful for achieving both anterior and lateral fluoroscopic images of the spine. The operating table should be capable of rotating in a side-to-side manner and tilting in a head up or down manner to allow the atelectatic lung to fall away from the region of the spine being accessed (Fig. 3–2). An axillary roll is placed as padding under the dependent axilla, and all other bony prominences are padded with foam or pillows. Given the possibility that the thoracoscopic procedure may be quite prolonged, careful attention must be paid to ensuring adequate padding and protection during the case. A sling or elevated armboard attachment can be used to keep the ipsilateral arm flexed at the shoulder and out of the way for access to the upper thoracic spine (Fig. 3–3). Adhesive tape, belts, or clamps should be used to secure the patient to the table and prevent motion when changing the table inclination. The thoracic region should be draped completely so that the procedure can be converted to an open thoracotomy if necessary. FIGURE 3–3 Lateral decubitus positioning for the patient undergoing thoracoscopy. Ensuring that the patient is in the direct lateral position will aid in fluoroscopic visualization. Attention should be paid to padding the arms, axilla, and bony prominences of the hip/pelvis and lower extremities adequately. To access the upper thoracic spine, the right arm can be abducted up and out of the way so that the portals can be inserted in a sufficiently cephalic position. (With permission from Rosenthal DJ, Dickman CA. Operating room setup and patient positioning. In: Dickman C, Rosenthal DJ, Perin NI, eds. Thoracoscopic Spine Surgery. New York: Thieme Medical Publishers; 1999:103.) In laparoscopy procedures, the patient is placed supine on a radiolucent table that can be tilted into a fairly steep reverse Trendelenburg position to allow for the abdominal contents to fall away from the lower lumbar spine. The arms may be tucked at the patient’s side. If this results in obstruction of fluoroscopic visualization, the shoulders may be abducted 90 degrees to move them up and away from the axial spine. When performing an anterior interbody procedure, the table can be extended if possible, or a large gel-pad can be placed under the lumbosacral junction in order to extend the lumbar spine focally. This will provide maximal access to the anterior disk space and will facilitate the restoration of normal sagittal alignment. Again, the patient should be secured to the table to prevent sliding when placed in the Trendelenburg position. Efficient staff and equipment positioning around the patient undergoing a minimally invasive procedure are absolute prerequisites prior to commencing surgery. In practice, the exact arrangement of the surgical personnel and equipment will be influenced by the actual procedure and the equipment availability. In thoracoscopy, a commonly used positioning arrangement is to have the spine surgeon and the first assistant standing in front of the patient, who is lying in the lateral decubitus position, with an additional assistant or scrub nurse standing behind the patient (Fig. 3–4). The primary surgeon controls the primary operating instruments (e.g., the rongeurs, burrs, and dissecting tools). The first assistant often operates the camera and helps with visualization, and the second assistant across the table often helps to retract the lung and provide suction. One video monitor should be placed toward the head of the bed, directly across the table from the primary surgeon so that the surgeon’s eyes and hands can be kept in a frontal position, obviating the need to turn his or her head while operating. A second monitor is placed toward the head of the bed on the surgeon’s side to be viewed by the assistant from the other side of the table. Surgical members committed to working an endoscopic instrument should have a video monitor placed as directly in front of them as possible. Similar principles exist for the arrangement of equipment and personnel for laparoscopic surgery. With the patient lying supine, the primary surgeon can choose to stand on either the right or left side of the patient based on familiarity, hand dominance, and side of pathology. The laparoscope camera is usually inserted into the superior aspect of the abdomen. The surgical assistant who holds the scope generally stands above the primary surgeon toward the head of the bed. The fluoroscopic unit is introduced from the side of the patient at the level of surgical pathology when needed. Video monitors should be positioned in a comfortable line of sight for the surgeon and the assistants. One monitor is usually positioned to the right or left of the patient in front of the primary surgeon’s line of sight. Another monitor is usually placed at the foot of the bed for efficient visualization by the assistants. FIGURE 3–4 two lumens and two occlusive balloons. Note that the video monitors are in the direct line of sight for the surgeon and assistant. The video monitor at the head of the bed may be used by the scrub assistant opposite the surgeon. In this configuration, the C-arm is brought in from the head of the bed, but alternatively it can come up from the foot. (With permission from Rosenthal DJ, Dickman CA. Operating room setup and patient positioning. In: Dickman C, Rosenthal DJ, Perin NI, eds. Thoracoscopic Spine Surgery. New York: Thieme Medical Publishers; 1999:96.) Endoscopic techniques to address spinal pathology will likely increase in popularity in the future. The anesthetic considerations of thoracoscopy and laparoscopy (e.g., the effects of single-lung ventilation and pneumoperitoneum) should be clearly appreciated by the spinal surgeon. Whereas the expectation of lowered morbidity will encourage surgeons to develop skills in endoscopic procedures, it should be recognized that the learning curve for these techniques is steep. Prior to undertaking such procedures, a sufficient degree of training must be obtained, and one must have a strong appreciation for the need to develop thoughtful strategies with regard to operating room setup and equipment requirements. REFERENCES 5. Fredman B. Physiologic changes during thoracoscopy. Anesthesiol Clin North Am. 2001;19:141–152. 6. Shah JS, Bready LL. Anesthesia for thoracoscopy. Anesthesiol Clin North Am. 2001;19:153–171. 15. Latham P, Dullye KK. Complications of thoracoscopy. Anesthesiol Clin North Am. 2001;19:187–200.
3

Anesthetic Considerations and Operating Room Setup for Minimally Invasive Endoscopic Spine Surgery

< div class='tao-gold-member'>
Anesthetic Considerations and Operating Room Setup for Minimally Invasive Endoscopic Spine Surgery
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








