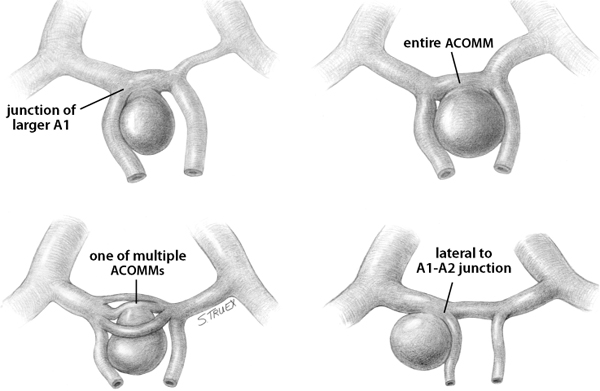
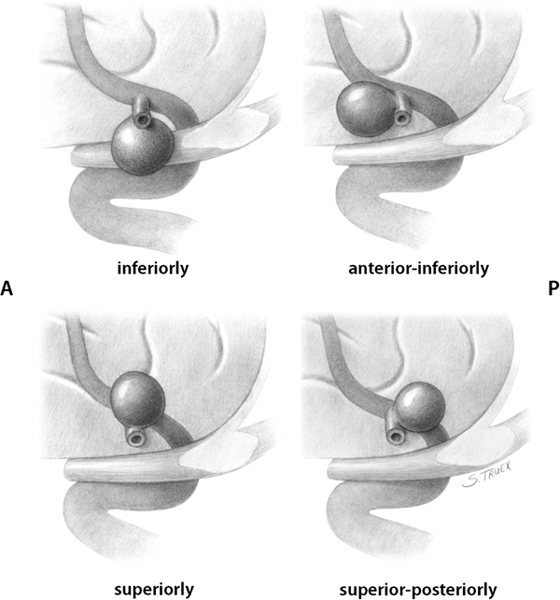
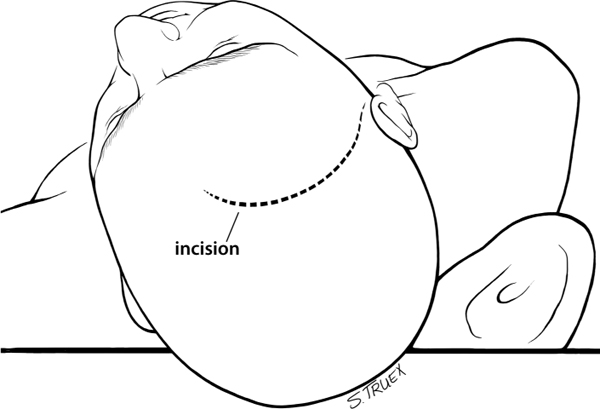
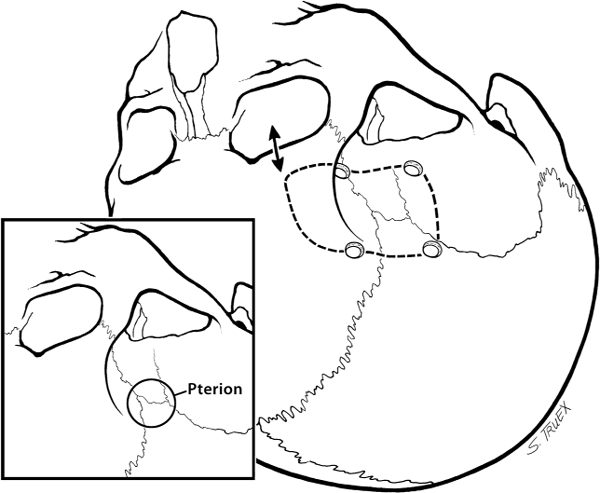
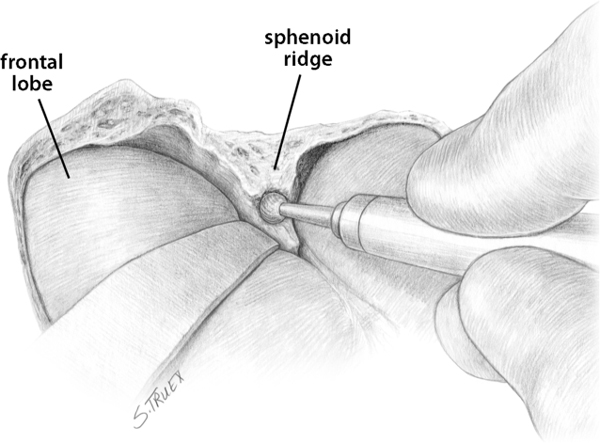
7 While often regarded as relatively simple anterior circulation lesions, these aneurysms can be deceptively difficult. Their interhemispheric location and close relationship to critical areas of brain function account for the significant morbidity and mortality associated with their rupture. Successful surgical treatment requires an understanding of the complex anatomy of the anterior communicating artery (ACOMM) complex and the multiple parent and branch arteries that must be identified and controlled prior to clip application. ACOMM aneurysms are the most common aneurysms associated with intracerebral bleeding. The hemorrhage pattern seen on computed tomographic (CT) scan is often characteristic, with varying amounts of hemorrhage in the subarachnoid space (prechiasmatic cistern and interhemispheric fissure), the brain parenchyma (gyrus rectus), and the ventricular system (lamina terminalis and third ventricle). As a general rule, ACOMM aneurysms arise at the junction of the larger A1 segment, the ACOMM, and the ipsilateral A2 segment origin. Exceptions to this rule are common (Fig. 7.1); these lesions may occupy the entire ACOMM, may arise on any one of several ACOMMs, and on occasion will arise from the lateral aspect of the A1–A2 junction abluminal from the anterior communicating origin. With the widespread use of both rotational angiography and CT angiography there are fewer intraoperative surprises awaiting the surgeon in terms of origin now than formerly, but we recommend a healthy dose of skepticism regarding the exact anatomy of the origin until it’s been conclusively demonstrated at surgery. Stubbornly trying to make the vasculature correspond to your (or someone else’s) interpretation of the imaging studies is a sure recipe for disaster when dealing with these complex anatomical situations. Fig. 7.1 Alternative anatomic configurations of ACOMM aneurysms. We normally think of the ACOMM as lying in the coronal plane, but actually, its orientation varies from coronal to truly sagittal, and preoperative awareness of this variability is important. A sagittal orientation of the communicating artery seems to be especially common when there is a large disparity in size between the two A1 segments and is most frequent when only a single A1 supplies both A2 segments. In this latter situation, the A2 origin ipsilateral to the large A1 has its origin in the interhemispheric fissure close to the optic chiasm, whereas the contralateral A2 exists as the distal continuation of the sagittally directed communicating artery. More on these specific aneurysms later. In addition to the orientation of the communicating artery, it’s important to consider the projection of anterior communicating aneurysms in both the sagittal and the coronal planes (Fig. 7.2). The most common sagittal (Fig. 7.2) projections are inferiorly into the prechiasmatic cistern or anteriorly into the subarachnoid space of the interhemispheric fissure. Less commonly, the aneurysm will project superiorly, and most uncommon of all is the rare superior-posterior projection. In the coronal plane, anterior communicating aneurysms may remain in the midline or jut laterally into one or the other gyrus rectus and mesial frontal lobe. Fig. 7.2 Projections of ACOMM aneurysms. The preceding information—orientation of the parent-communicating artery, probable site of origin of the aneurysm, and plane of projection of the aneurysm’s dome—is helpful in defining the laterality of operative approach and the sequence of events in dissection of the afferent and efferent vasculature. In general, assuming that both patient and surgeon are left-hemisphere dominant, we think the majority of anterior communicating aneurysms are best treated from a right-sided approach. Dissection and clip application are somewhat easier for a right-handed surgeon approaching the anterior aspect of the circle of Willis from the right; similarly, the risk of serious neurological injury is somewhat reduced if the necessary brain retraction involves the patient’s nondominant frontal cortex. Regardless of approach, both A1 segments must be isolated for adequate proximal control; thus the laterality of the dominant A1 is generally irrelevant in determining the optimal side of operative exposure. Exceptions to these general rules are few but important; in the small number of patients in whom the aneurysm appears to arise from the lateral aspect of the A1–A2 junction and in those more common cases that harbor a large gyrus rectus hematoma, the procedure will proceed more smoothly if the surgeon has direct access to the aneurysm origin in the first situation and to the clot itself in the second. Finally, when the anterior communicating aneurysm is only one of multiple aneurysms undergoing treatment, obviously the laterality of approach should be selected to facilitate obliteration of the ruptured lesion, if one can be identified, and second to maximize the number of aneurysms that can be treated safely via a single exposure. Regardless of the operative side, the patient is placed supine with the neck extended and the head rotated away from the surgical site, then tilted to bring the floor of the frontal fossa roughly perpendicular to the long axis of the patient’s body (Fig. 7.3). Normally, for a right-sided approach the head is rotated ~45 to 60 degrees; when operating from the left we prefer a full 60 degrees of rotation. These aneurysms can be successfully approached via an interhemispheric exposure, a lateral subfrontal route, or a “modified” transsylvian approach that morphs into a subfrontal exposure once the carotid bifurcation has been reached. Fig. 7.3 Skin incision for ACOMM aneurysm. This latter approach has been used at University of Texas Southwestern (UTSW) for most anterior communicating aneurysms over the past 30 years; it works equally well in small and giant lesions, in the slack brains of patients with unruptured aneurysms, and in the hot, swollen brains of extremely sick subarachnoid hemorrhage victims. This exposure offers immediate access to the ventricular system via the Payne point, and with very little modification can be used to expose lesions in the sagittal plane from the basilar apex to the ACOMM. It’s the best approach for young surgeons to safely learn the vascular anatomy of the anterior circle of Willis and is obviously the exposure of choice if more than one aneurysm is to be treated at a single sitting. Currently about two thirds of our anterior communicating aneurysms are approached in this fashion. When compared with the bony exposure used routinely for aneurysms of the middle cerebral, internal carotid, and basilar arteries, this craniotomy is centered slightly more anteriorly (extending to the midpupillary line) and exposes less of the temporal lobe, as the posterior burr hole is placed only slightly posterior to the pterion (Fig. 7.4). No subtemporal craniotomy is done, and the sphenoid ridge is only flattened, rather than being radically resected with the burr (Fig. 7.5). The inferior aspect of the craniotomy is carried as low as humanly possible without opening the bony orbit, and then the inner table of the skull along the inferior margin of the craniotomy defect is aggressively drilled away to effectively lower the inferior limit of exposure to the level of the orbital roof. After a small amount of experimentation with orbitotomy, we felt it honestly provided little or no improvement in the ultimate exposure and have not subsequently relied on this additional bone removal. It’s a little bit like a swagger stick; if you need one, by all means use it, but most folks can learn to do without. Fig. 7.4 Craniotomy for ACOMM aneurysm. Fig. 7.5 Extradural drill resection of sphenoid ridge. The arachnoid overlying the most inferior aspect of the cortical portion of the sylvian fissure is identified and opened sharply to expose the insula and M2 segments of the middle cerebral artery. Dissection then proceeds from laterally to medially, opening the sphenoidal portion of the fissure to expose the middle cerebral bifurcation and then the M1 segment. Retractors are not placed on the frontal and temporal opercula unless the brain is swollen; generally, the entire fissure can be opened to the carotid cistern, using the microscissors and suction tube to gently separate the two lobes as the arachnoid binding them is cut sharply. However, if necessary, the retractor can be placed on the parenchyma overlying the carotid-ophthalmic cistern (Fig. 7.6). When the origin of the A1 segment is identified (Fig. 7.7), a thin, self-retaining retractor blade can be situated on the posterior orbital cortex immediately anterior to and parallel with the sylvian fissure. The tip of the blade should extend to the carotid bifurcation and expose the initial millimeters of the anterior cerebral artery. The A1 segment is circumferentially dissected at this point for temporary clip application if necessary. Then, by cutting the arachnoid fibers holding the gyrus rectus to the optic nerve, the cistern of the A1 can be opened and the artery followed anteriorly and medially across the nerve toward the prechiasmatic cistern.
Aneurysms of the Anterior Communicating Artery
General
Anatomy
Projections
Procedure
Approach
Positioning
Craniotomy
Initial Approach
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree