Chapter 22 Anterior endoscopic cervical microdecompression of disc and foramen
The standard treatment for cervical disc protrusions and foraminal stenosis ha s been anterior cervical microdecompression of the disc and foramen with or without bony fusion [1–5]. These open operations are associated with significant local morbidity [6,7], such as graft collapse, graft extrusion, hardware failure, nonfusion with resultant instability, infections, esophageal perforation with infection, and permanent pain, peripheral nerve injury, or infection at the graft donor site. Anterior cervical fusion (ACF) is associated with a 15% or greater chance of junctional disc herniation or adjacent segment disease at interspaces adjacent to fused levels [8,9].
The evolution of spinal surgery is trending toward less invasive techniques [9–16]. Advancements in microinstrumentation, fiberoptics, improved fluoroscopic imaging, and high-resolution digital video imaging endoscopy, along with the accumulation of experience in percutaneous lumbar discectomy [17–20] and spinal laser applications [20–23], have facilitated the development of anterior endoscopic cervical microdecompression (AECM) and foraminal decompression [9,14,19]. AECM, as minimally invasive surgery, does not affect the stability of adjacent vertebral segments [8–10]. Although ACF is often an unattractive treatment for patients with multiple-level disc symptoms, AECM can be safely utilized for treatment of such patients.
Treatment objectives
The primary objective of AECM is to perform decompression of the herniated cervical disc and foraminal disc. It is a minimally invasive outpatient procedure that aims to reduce tissue trauma with much less morbidity than open cervical spinal surgery [9,10,12]. There is no graft donor site to cause secondary problems, and the period of convalescence and the costs of the procedure are significantly less than those of traditional open operations.
Indications
The indications for AECM are as follows [9,12,19]:
 Physical findings of sensory loss, muscle weakness, and/or decreased reflexes in the upper extremities that correlate with the level of involvement
Physical findings of sensory loss, muscle weakness, and/or decreased reflexes in the upper extremities that correlate with the level of involvement Magnetic resonance imaging (the imaging study of choice) or computed tomography findings of disc herniation consistent with the dermatome of clinical symptoms
Magnetic resonance imaging (the imaging study of choice) or computed tomography findings of disc herniation consistent with the dermatome of clinical symptoms Junctional disc herniation syndrome (JDHS) or adjacent segmental disease (ASD) in patients who have undergone cervical fusion
Junctional disc herniation syndrome (JDHS) or adjacent segmental disease (ASD) in patients who have undergone cervical fusionContraindications
Contraindications to AECM are as follows [9,12]:
 Advanced spondylosis (significant bone spurs) with severe disc space narrowing and with osteophytes blocking entry into the disc space.
Advanced spondylosis (significant bone spurs) with severe disc space narrowing and with osteophytes blocking entry into the disc space.Advantages
The advantages of AECM in comparison with open procedures are as follows [8,9,12,19,22]:
 No dissection of muscle, bone, ligaments, or manipulation of the dural sac or nerve roots; little or no epidural bleeding
No dissection of muscle, bone, ligaments, or manipulation of the dural sac or nerve roots; little or no epidural bleedingInstrumentation
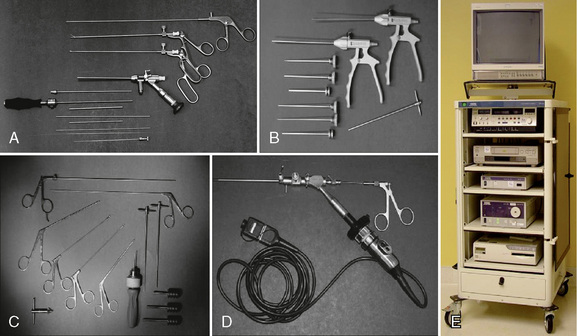
The following instruments and equipment are needed for AECM (Fig. 22-1) [9,12]:
 Endoscopic tower equipped with digital video monitor, DVT/VHS recorder, light source, photo printer, and tri-chip digital camera system.
Endoscopic tower equipped with digital video monitor, DVT/VHS recorder, light source, photo printer, and tri-chip digital camera system. Cervical endoscopic discectomy set (Karl Storz, Tuttlingen, Germany), including 4-mm, 0-degree endoscope.
Cervical endoscopic discectomy set (Karl Storz, Tuttlingen, Germany), including 4-mm, 0-degree endoscope. Cervical discectomy sets (2.5 and 3.5 mm) (Blackstone Medical, Inc., Springfield, MA) with short cervical discectomes.
Cervical discectomy sets (2.5 and 3.5 mm) (Blackstone Medical, Inc., Springfield, MA) with short cervical discectomes. More aggressively toothed trephines used for spurs and spondylitic ridges at the anterior and posterior disc space.
More aggressively toothed trephines used for spurs and spondylitic ridges at the anterior and posterior disc space. Holmium:yttrium-aluminum-garnet (Ho:YAG) laser generator (Trimedyne, Inc., Irvine, CA) with right-angle (side-firing) probe (Fig. 22-2).
Holmium:yttrium-aluminum-garnet (Ho:YAG) laser generator (Trimedyne, Inc., Irvine, CA) with right-angle (side-firing) probe (Fig. 22-2).Procedure
Patient Positioning
Figure 22-3 illustrates patient positioning for AECM [9,12]:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree