FIGURE 3.1 The neurocognitive network for language. The core perisylvian language cortices lie within the dashed line and include Broca’s area in the inferior frontal gyrus, the supramarginal- and angular gyri in the parietal lobe, the subjacent arcuate fasciculus white matter tract, and Wernicke’s area in the superior temporal gyrus. Extrasylvian sites that produce transcortical aphasias are found in surrounding cortices (beyond dashed line). (Modified with permission from Mayeux R, Kandel ER. Disorders of language: the aphasias. In: Kandel ER, Schwartz JH, Jessell TM, eds. Principles of Neural Science. 3rd ed. New York, NY: Elsevier; 1991.)
A. Stroke. Cerebrovascular disease is a frequent cause of aphasia. The perisylvian language zone is supplied by divisions of the middle cerebral artery (MCA), a branch of the internal carotid artery (ICA). The classic aphasic syndromes are most distinctly observed in ischemic stroke because vascular occlusions produce discrete, well-delineated brain lesions.
B. Other focal lesions. Any focal lesion affecting the language cortices will also produce aphasia, including primary and metastatic neoplasms and brain abscesses. Primary progressive aphasia (PPA) is a neurodegenerative syndrome characterized by slowly progressive, isolated language impairment in late life and focal atrophy of dominant frontotemporal cortices. Affected individuals frequently develop a generalized dementia after the first 2 years of illness. The three main variants of PPA and their anatomical correlate are as follows: (a) nonfluent/agrammatic (left posterior frontal and insular regions); (b) semantic (left anterior temporal); and (c) logopenic (left temporoparietal region). Among the causes of PPA are (1) tau-positive, ubiquitin/TDP 43-positive frontotemporal lobar degeneration and (2) a focal variant of Alzheimer’s disease (AD) pathology.
C. Diffuse lesions. Diseases producing widespread neuronal dysfunction will disrupt language processing along with other cognitive and noncognitive neural functions. Traumatic head injury and AD are epidemiologically common causes of aphasic symptoms, although not of isolated aphasia.
CLINICAL MANIFESTATIONS
A. Nonfluency versus fluency. Fluency refers to the rate, quantity, and ease of speech production. In nonfluent speech, verbal output is meager (<50 words per minute), phrase length shortened (one to four words per phrase), production effortful, articulation often poor, and the melodic contour (prosody) disturbed. Nonfluent speakers often preferentially employ substantive nouns and verbs, eliding small connecting grammatical/functor words (“telegraphic speech”). Conversely, in fluent speech, verbal output is generous (and may even be more abundant than customary), phrase length normal, production easy, articulation usually preserved, and the melodic contour intact.
1. Anatomic correlate. Nonfluency indicates damage to the frontal language regions anterior to the fissure of Rolando. Fluency signals that these areas are intact.
B. Auditory comprehension impairment. Impaired ability to understand spoken language ranges from complete mystification by simple one-word utterances to subtle failure to extract the full meanings of complex sentences. In informal conversation, aphasic patients often capitalize on clues from gestures, tone, and setting to supplement their understanding of the propositional content of a speaker’s utterances. Examiners may underestimate the extent of auditory comprehension impairment if they fail to test formally a patient’s comprehension deprived of nonverbal cues.
1. Anatomic correlate. Comprehension impairment generally reflects damage to the temporoparietal language regions posterior to the fissure of Rolando. Preserved comprehension indicates that these areas are intact. (Comprehension of grammar is an important exception to this rule. Agrammatism is associated with damage to inferior frontal language regions.)
C. Repetition impairment. Repetition of spoken language is linguistically and anatomically a distinct language function. In most patients, repetition impairment parallels other deficits in spoken language. Occasionally, however, relatively isolated disordered repetition may be the dominant clinical feature (conduction aphasia). In other patients, repetition may be well preserved despite severe deficits in spontaneous speech (transcortical aphasias). Rarely, such patients exhibit echolalia, a powerful, mandatory tendency to repeat all heard phrases.
1. Anatomic correlate. Impaired repetition indicates damage within the core perisylvian language zone. Preserved repetition signals that these areas are intact.
D. Paraphasic errors. Substitutions of incorrect words for intended words are paraphasias. Paraphasic errors are classified into three types.
1. A literal or phonemic paraphasia occurs when only a part of the word is misspoken, as when “apple” becomes “tapple” or “apfle.”
2. A verbal or global paraphasia occurs when an entire incorrect word is substituted for the intended word, as when “apple” becomes “orange” or “bicycle.” A semantic paraphasia arises when the substituted word is from the same semantic field as the target word (“orange” for “apple”). Fluent output contaminated by many verbal paraphasias is jargon speech.
3. A neologistic paraphasia occurs when an entirely novel word not extant in the speaker’s native lexicon is substituted for the intended word, as when “apple” becomes “brifun.”
4. Anatomic correlate. Paraphasic errors may occur with lesions anywhere within the language system and do not carry strong anatomic implications. To some extent, phonemic paraphasias are more common with lesions in the frontal language fields and global paraphasias more common with lesions in temporoparietal areas.
E. Word-finding difficulty (anomia). Retrieval of target words from the lexicon is virtually always disturbed in aphasia. Patients may exhibit frequent hesitations in their spontaneous speech while they struggle with word finding. Circumlocutions transpire when patients “talk around” words they fail to retrieve, providing lengthy definitions or descriptions to convey the meanings of words they are unable to access.
1. Anatomic correlate. Word-finding difficulty occurs with lesions located throughout the language-dominant hemisphere and possesses little localizing value.
F. Reading and writing. In most cases of aphasia, reading impairment (alexia) and writing impairment (agraphia) parallel oral language comprehension and production deficits. Occasionally, however, isolated reading impairment, writing impairment, or both can occur in the setting of fully preserved oral language function.
1. Anatomic correlate. The anatomy of reading and writing incorporates both the core perisylvian language zones and additional function-specific sites. Reading requires primary and higher-level visual processing in the occipital and inferior parietal lobes. Writing depends on visual stores in the inferior parietal lobe and graphomotor output regions in the frontal lobe.
EVALUATION
A. History. Abrupt onset of language difficulty suggests a cerebrovascular lesion. Subacute onset may suggest tumor, abscess, or other more moderately progressive process. Slow onset suggests a degenerative disease, such as AD or frontotemporal lobar degeneration. Interviewing family members and other observers is crucial when the patient’s language difficulty limits direct history-taking.
B. Physical examination.
1. Elementary neurologic signs. A detailed elementary neurologic examination allows identification of motor, sensory, or visual deficits that accompany the language disorder, aiding neuroanatomic localization. Important “neighborhood” signs are the presence or absence of hemiparesis, homonymous hemianopia or quadrantanopia, and apraxia.
2. Mental status examination. It is important to assess the patient’s wakefulness and attentional function lest language errors resulting from inattentiveness be wrongly ascribed to intrinsic linguistic dysfunction. Nonverbal tests to evaluate memory, visuospatial, and executive functions should be used if severe language disturbance precludes routine verbal assessment.
3. Language examination. A careful language examination is critical in the evaluation of aphasia, profiling the patient’s impaired and preserved language abilities and allowing a syndromic, localizing diagnosis (Video 3.1).![]()
a. Spontaneous speech. The patient’s spontaneous verbal output, in the course of conversation and in response to general questions, should be judged for fluency versus nonfluency and presence or absence of paraphasias. It is important to ask open-ended questions such as “Why are you in the hospital?” or “What do you do during a typical day at home?” because patients may mask major language derangements with yes/no answers and other brief replies to more structured interrogatories.
b. Repetition. The patient is asked to repeat complex sentences. If difficulty is evidenced, simpler verbal sequences from single-syllable words to multisyllabic words and short phrases are given to determine the level of impairment. At least one sentence rich in grammatical/functor words, such as “No ifs, ands, or buts,” should be employed to test for isolated or more pronounced difficulty in grammatical repetition, as may be seen in Broca’s and other anterior aphasias.
c. Comprehension. An initial judgment of auditory comprehension can be made in the course of obtaining the medical history and from spontaneous conversation. Tests that require no or minimal verbal responses are essential to the evaluation of auditory comprehension in individuals with severe disturbance of speech production and intubated patients.
(1) Commands. One simple bedside test is verbally to instruct the patient to carry out one-step and multistep commands, such as “Pick up a piece of paper, fold it in half, and place it on the table.” Cautions to recall when interpreting results are (a) apraxia and other motor deficits may cause impairment not related to comprehension deficit and (b) midline motor acts on command, such as closing/opening eyes and standing up, draw on distinct anatomic systems and may be preserved even in the setting of severe aphasic comprehension disturbance.
(2) Yes/no responses. If the patient can reliably produce verbal or gestural yes/no responses, this output system may be used to assess auditory comprehension. Questions of graded difficulty should be employed for precise gauging of the degree of comprehension disturbance, using queries ranging from simple (“Is your name Smith?”) to complex (“Do helicopters eat their young?”).
(3) Pointing. This simple motor response also permits precise mapping of comprehension impairment by means of questions of graded difficulty. The examiner should employ both simple pointing commands (“Point to the chair, nose, door”) and more lexically and syntactically complex pointing commands (“Point to the source of illumination in this room”).
d. Naming. Difficulty with naming is almost invariable in all the aphasia syndromes. Consequently, naming tasks are sensitive, although not specific, means of testing for the presence or absence of aphasia.
(1) Confrontation naming. The patient is asked to name objects, parts of objects, body parts, and colors pointed out by the examiner. Common, high-frequency words (“tie,” “watch”) and uncommon, low-frequency words (“knot” of the tie, “watchband”) should be tested.
(2) Word-list generation. Another type of naming test is to ask the patient to generate a list of items in a category (animals, cars) or words beginning with a given letter (F, A, S). Normal individuals produce 12 or more words per letter in 1 minute.
e. Verbal automatisms. Patients with profound disruptions of speech production should be requested to produce (a) overlearned verbal sequences, including the numbers from 1 to 10 and the days of the week; (b) overlearned verbal material, such as the pledge of allegiance; and (c) singing, such as “Happy Birthday to You.” These utterances draw on subcortical and nondominant hemisphere areas and indicate residual capacities in impaired patients that may be capitalized on in rehabilitation.
f. Reading. Patients should be asked to read sentences aloud. Written sentences that are commands (“Close your eyes”) allow simultaneous testing of reading aloud and reading comprehension.
g. Writing. In the order of difficulty, patients may be asked to write single letters, words, and short sentences. Obtaining a signature is insufficient because this overlearned sequence may be retained when all other graphomotor functions are lost.
C. Laboratory studies.
1. Computed tomography. Head computed tomography (CT) scan will delineate most focal structural lesions affecting the language regions of the brain. It may be normal in the first 24 hours following acute aphasia from new-onset ischemic stroke.
2. Magnetic resonance imaging. Brain magnetic resonance imaging (MRI) is somewhat more sensitive than CT at detecting morphologic abnormalities and is the preferred study if readily available. Imaging in the sagittal and coronal as well as axial planes allows precise mapping of lesions within known neural language regions.
Distinctive features of a patient’s language disturbance may be employed to assign a syndromic diagnosis that has localizing value. Eight classical cortical aphasia syndromes are distinguished on the basis of fluency, comprehension, and repetition (Fig. 3.2). Approximately 60% of all aphasic patients exhibit one of these symptom clusters. Most of the remaining “atypical” aphasias will be found to harbor subcortical lesions. It is important to consider the time after onset when employing these syndromes for clinicoanatomic correlation. Soon after an acute insult, deafferentation, edema, hypoperfusion without infarction, and other mechanisms of diaschisis produce exaggerated clinical deficits. Later, neuroplasticity-mediated recovery of function reduces clinical deficits. The aphasia syndromes have maximal localizing value 3 weeks to 3 months after onset.
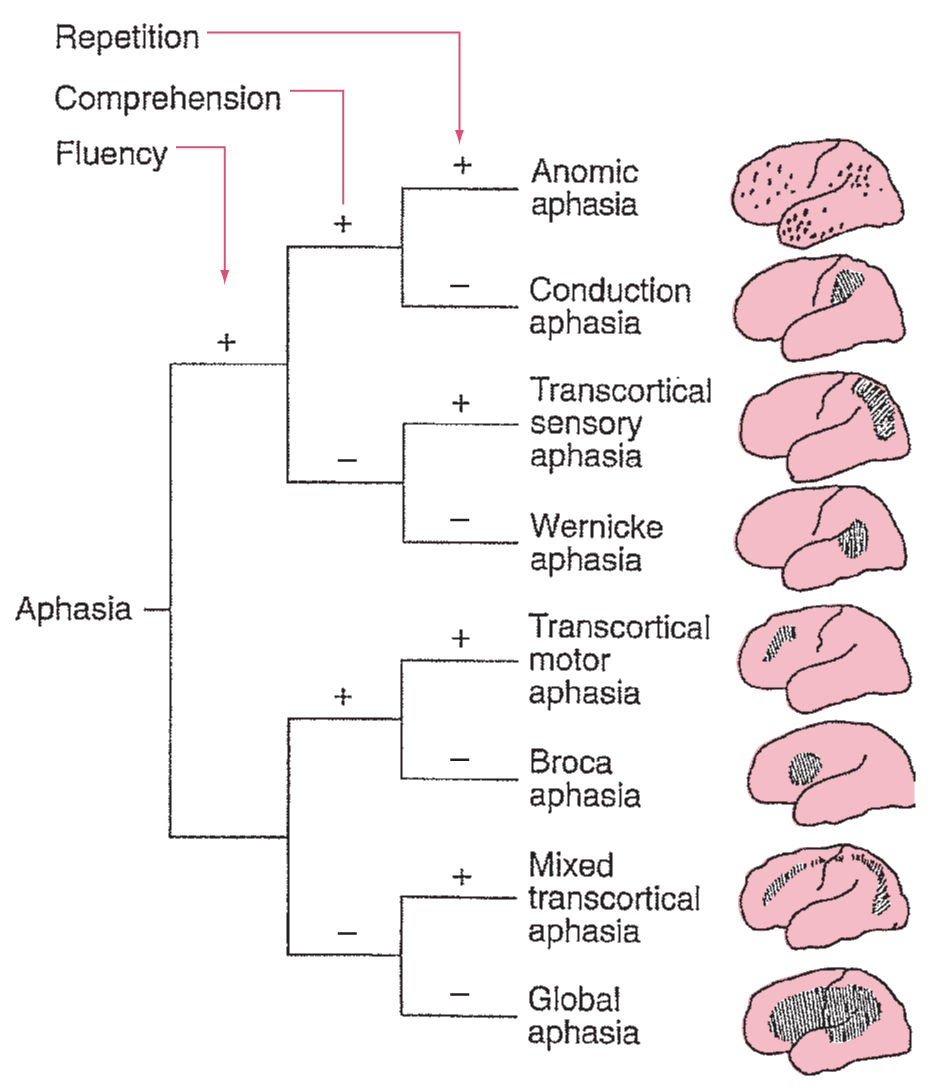
FIGURE 3.2 Algorithm for diagnosis and localization of the eight classical cortical aphasias.
A. Perisylvian aphasias.
1. Broca’s aphasia. Patients with Broca’s aphasia exhibit (a) nonfluent, dysarthric, effortful speech; (b) similarly disordered repetition; and (c) relatively intact comprehension, with mild difficulty in understanding syntax and relational grammar. Their verbal output is often “telegraphic,” containing substantive nouns and verbs but omitting small, connecting, functor words. Most patients exhibit a faciobrachial hemiparesis. Patients often exhibit frustration over their language deficits and are at elevated risk for depression.
a. Lesions producing Broca’s aphasia lie in the posterior portion of the inferior frontal gyrus (Broca’s area; Brodmann areas 44 and 45) and extend to involve surrounding motor, premotor, and underlying white matter territories. Lesions restricted solely to Broca’s area produce mild, transient aphasia and more persistent dysarthria.
b. Broca’s area is supplied by the superior division of the MCA.
2. Wernicke’s aphasia. Patients with Wernicke’s aphasia evince fluent, effortless, well-articulated output, almost always contaminated with paraphasias and neologisms. Repetition demonstrates a parallel impairment, with fluent but paraphasic output. The leading feature of Wernicke’s aphasia is a severe disturbance of auditory comprehension. Two types of behavioral responses to this comprehension deficit are observed. Most often in the acute phase, patients seem to be unaware of their inability to comprehend spoken language, calmly providing inappropriate and grossly paraphasic answers to observer’s inquiries. Less frequently, patients are irritable and paranoid, perhaps because of their inability to understand what others say. A superior homonymous quadrantanopia is frequently present. However, the absence of more dramatic motor or sensory deficits, and the fluid production of speech, may mislead medical personnel into believing that the patient is confused or psychotic rather than aphasic, and may delay diagnosis while metabolic or psychiatric disturbances are sought.
a. The core of lesions engendering Wernicke’s aphasia map to the posterior third of the superior temporal gyrus (Wernicke’s area; Brodmann area 22), an auditory association area. Lesion size may vary considerably, and damage often extends to the middle temporal gyrus and the inferior parietal lobe.
b. Wernicke’s area is supplied by the inferior division of the MCA.
3. Global aphasia. The most profound form of aphasia, called global aphasia, is characterized by drastically nonfluent output, severe disruption of comprehension, and little repetitive ability. Spontaneous speech is often absent initially, or marked by the production of a few stereotyped sounds. Patients neither read nor write. Hemiplegia is almost invariably present, and hemisensory loss and hemianopia are frequent.
a. The typical insult involves the entire left perisylvian region, encompassing Broca’s area in the inferior frontal lobe, Wernicke’s area in the posterior temporal lobe, and all the interposed parietofrontal cortices. In rare cases, separate, discrete lesions of Broca’s area and Wernicke’s area produce global aphasia without hemiparesis.
b. The perisylvian region lies within the territory of the MCA, and ICA and MCA occlusions are the most common causes of global aphasia.
4. Conduction aphasia. The hallmark of conduction aphasia is a disproportionate disruption of repetition. Comprehension of spoken language is relatively intact. Fluent spontaneous output is often marred by occasional hesitations and phonemic paraphasias, but is not as disturbed as repetition. Naming also tends to demonstrate mild paraphasic contamination. Motor and sensory disturbances are usually absent or mild.
a. Two neural loci tend to give rise to conduction aphasia: (a) the supramarginal gyrus, sometimes with extension to the subinsular white matter, and (b) the primary auditory cortex, insula, and subjacent white matter. The arcuate fasciculus, a subcortical white matter tract connecting Wernicke’s and Brodmann’s areas, is often, but not invariably, involved.
b. These regions are variably supplied by branches of the inferior or superior divisions of the MCA.
B. Extrasylvian aphasias. The extrasylvian aphasic syndromes share the clinical characteristic of preserved repetition and the anatomic trait of sparing of the core perisylvian language zone. They occur less commonly than the perisylvian aphasias. Many arise from watershed infarcts, but they may also appear in conjunction with tumors, abscesses, hemorrhages, and other lesions.
1. Transcortical motor aphasia. Transcortical motor aphasia is characterized by discrepant spontaneous speech and repetition. Spontaneous output is severely disrupted, nonfluent, and halting. In contrast, the ability to repeat sentences verbatim is preserved, as is reading aloud. Comprehension is undisturbed. Naming may be mildly impaired.
a. Transcortical motor aphasia results from damage at one of two foci: (a) prefrontal cortices and subjacent white matter anterior or superior to Broca’s area or (b) the supplementary motor area and cingulate gyrus. These lesions disconnect Broca’s area from limbic areas and other sources of the drive to communicate.
b. Lesions anterosuperior to Broca’s area lie in the vascular border zone between the middle and anterior cerebral arteries. The supplementary motor area and cingulate gyrus regions are irrigated by the anterior cerebral artery (ACA).
2. Transcortical sensory aphasia. Patients with transcortical sensory aphasia exhibit severely disturbed comprehension of spoken language, but preserved repetition. Spontaneous speech is fluent, although often paraphasic. Echolalia—automatic repetition of overheard phrases—is common. Reading aloud may be fairly preserved, whereas reading comprehension is quite poor. Motor deficits are generally absent, but hemisensory deficits are common.
a. Lesions may occur over a wide distribution posterior and superior to the posterior perisylvian region, including the middle temporal gyrus, the angular gyrus, and underlying white matter. These insults disconnect Wernicke’s area from multiple posterior association cortices, preventing retroactivation by aural word forms of the widely distributed neural representations that convey their meanings.
b. The lesions generally lie within the vascular watershed between the posterior cerebral artery (PCA) and MCA.
3. Mixed transcortical aphasia. This rare and remarkable condition is analogous to global aphasia, except for preserved ability to repeat. Spontaneous speech is minimal or absent. Patients are unable to comprehend spoken language, name, read, or write. Repetition of spoken language, however, is preserved. Patients are often echolalic. Mild hemiparesis and hemisensory loss affecting proximal greater than distal extremities may be observed.
a. Lesions are an additive combination of those producing transcortical motor and sensory aphasias. Insults anterosuperior to Broca’s area and posterosuperior to Wernicke’s area cut off the perisylvian language zone from access to other cortices. Isolation of the speech area is a synonym for mixed transcortical aphasia.
b. The lesions fall in the crescentic vascular border zone among the ACA, MCA, and PCA.
4. Anomic aphasia. These patients exhibit difficulty retrieving verbal tags in spontaneous speech and confrontation naming. The remainder of language functions is relatively intact. Auditory comprehension, repetition, reading, and writing are normal. Spontaneous speech is preponderantly fluent, although interrupted by occasional hesitations for word finding. In severe cases, output may be lengthy but empty, with recurrent circumlocutions.
a. A wide variety of lesions, including both dominant and nondominant hemisphere loci, may produce anomic aphasia. Particularly common sources are insults to (a) the dominant inferior parietal lobe and (b) the dominant anterior temporal cortices. The latter insults have been associated with category-specific naming deficits in which naming in different semantic categories (e.g., living versus nonliving entities) is differentially impaired.
b. The angular gyrus and anterior temporal cortices are supplied by different branches of the inferior division of the MCA.
C. Subcortical aphasia syndromes. Focal lesions confined to subcortical structures strongly interconnected with language cortices produce aphasia. Although the optimal classification system for the subcortical aphasias is a still contested and unsettled enterprise, two major profiles can be discerned according to neuroanatomic location of the lesions.
1. Striatal-capsular aphasia. The language deficit in striatal-capsular aphasia resembles that in anomic or transcortical motor aphasia. Patients may or may not be fluent but are almost invariably dysarthric. Mild to moderate anomia coexists with generally intact auditory comprehension, repetition, reading, and writing. Generation of complex syntactic sentences is impaired. Hemiparesis is common, hemisensory loss variable, and hemianopia infrequent. Lesions involve the left putamen, dorsolateral caudate, anterior limb of the internal capsule, and rostral periventricular white matter. This aphasia has been associated with both ischemic and hemorrhagic strokes.
2. Thalamic aphasia. The language deficit in thalamic aphasia resembles that in transcortical sensory aphasia. Output may be relatively fluent, auditory comprehension is deficient, and repetition is preserved. Impairments of naming, reading comprehension, and writing are also present. A contralateral emotional facial paresis (diminished facial movement in expressing spontaneous emotions but preserved facial movements to command) and contralateral hypokinesia are often the only elementary neurologic deficits. Lesions are situated in the left anterolateral thalamus. Thalamic aphasia has been associated with left thalamic infarction often involving the left tuberothalamic artery territory, and left thalamic hemorrhage.
D. Additional classical syndromes. Strategically placed lesions may produce dissociated impairments of reading, writing, and oral language function. Three syndromes with well-characterized localizing properties will be reviewed.
1. Alexia without agraphia (pure alexia). Alexia without agraphia, the first of the disconnection syndromes described by Joseph Jules Déjerine in 1892, presents as an acquired loss of reading ability in a literate person, with preserved ability to write spontaneously. Reading is severely impaired, whereas spontaneous speech, repetition, and auditory comprehension are normal. Writing is preserved, but dramatically, after a delay, patients are unable to read phrases they themselves have written. Recognition of words spelled aloud and traced on the palm is normal. Only words presented visually pose difficulty. Patients frequently exhibit a slow, letter-by-letter reading strategy, painstakingly recognizing and stating aloud each letter in a word and then, from the string of spoken letters, determining the target word. A right homonymous hemianopia is common but not invariable. Disorders of color vision, including achromatopsia and color anomia, may be present.
The most common neuroanatomic substrate comprises simultaneous lesions of the left occipital lobe and the splenium of the corpus callosum, depriving the angular gyrus region critical for word recognition of visual input from either the left or right hemisphere. The smallest sufficient injury is a single lesion of the paraventricular white matter of the mesial occipitotemporal junction (the forceps major), interrupting interhemispheric and intrahemispheric visual tracts to the angular gyrus but sparing the corpus callosum and left occipital cortex. Etiologies include left PCA infarction, tumor, demyelinating disorders such as multiple sclerosis (MS) or acute disseminated encephalomyelitis (ADEM), toxoplasma or herpes encephalitis, and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS).
2. Alexia with agraphia. Patients exhibit loss of literacy—inability to read or write—but relatively well-preserved oral language function. Speech is fluent, although anomia is often present, and auditory comprehension and repetition are intact. Hemisensory deficits are frequent, and hemiparesis and hemivisual disturbances are variable. A full-fledged Gerstmann’s syndrome, including dyscalculia, dysgraphia, left–right confusion, and finger agnosia, may be present.
The underlying lesion classically involves the dominant inferior parietal lobule (angular and supramarginal gyri).
3. Pure word deafness (auditory verbal agnosia). Patients resemble Wernicke’s aphasics. Comprehension and repetition of spoken language are impaired, whereas speech is fluent. Unlike in Wernicke’s patients, however, paraphasias are rare and, more importantly, comprehension of written material is intact. Writing production is also normal. Although uncomprehending of word sounds, patients have intact hearing and are generally successful in identifying meaningful nonverbal sounds such as car horns or telephone rings.
Two types of lesions underlie pure word deafness, both disconnecting Wernicke’s area from input from primary auditory cortices. Some patients harbor bilateral superior temporal lesions. A roughly equal number exhibit a single deep superior temporal lesion in the dominant hemisphere, blocking ipsilateral and crossing callosal auditory pathways.
E. Aprosodia. Meaning is conveyed not only through the propositional content of language, but also through prosody—the melody, rhythm, timbre, and inflection of the speaker. Prosody is frequently disturbed in nonfluent aphasia. However, patients may have normal propositional language yet exhibit disturbances of the production, comprehension, or repetition of prosody. Both cortical and subcortical dysfunctions can account for impaired prosody. In general, the nondominant hemisphere (most often the right) plays a greater role in production and comprehension of emotional prosody than does the dominant hemisphere.
DIFFERENTIAL DIAGNOSIS
Acquired speech impairments may result from disruption of lower-order neural and muscular mechanisms for implementing sound production rather than disturbances of central processing of language. It is important to distinguish these nonaphasic speech impairments from genuine aphasia, because they differ in their localizing significance and spectrum of etiologic causes.
A. Dysarthria. Dysarthria is abnormal articulation of spoken language. At least five types of nonaphasic dysarthria may be distinguished. (a) Paretic dysarthria is caused by weakness of articulatory muscles. Soft, low-pitched, nasal voicing is characteristic. Causes include myopathies, neuromuscular junction disorders such as myasthenia gravis, and lower motor neuron disease. (b) In spastic dysarthria, speech is typically strained, slow, and monotonic. Bilateral upper motor neuron lesions compromising the corticobulbar tracts are the cause. (c) In ataxic dysarthria, jerky irregular speech rhythm and volume are noted, reflecting lesions to the cerebellum or its connections. MS is a common cause. (d) Extrapyramidal dysarthrias include hypokinetic dysarthria, which is seen in parkinsonism and choreic dysarthria, which is observed in Huntington’s disease and other chorea syndromes. (e) In aphemia (apraxia of speech), small lesions within Broca’s area (Brodmann areas 44 and 45) or the left frontal oral motor cortex near the face M1 area produce dysarticulation without disturbing core language function.
Aphasic dysarthria—dysarticulation occurring as one manifestation of an aphasic language syndrome—is common with anterior aphasias such as Broca’s syndrome. The nonaphasic dysarthrias may be distinguished from aphasic dysarthria by demonstrating preserved intrinsic language functions including naming, comprehension, and reading. Intact writing is most telling, showing normal productive language capacity when a nonoral output channel is employed.
B. Mutism. Aphasia—disordered language—can be securely diagnosed only on the basis of exemplars of disturbed output (or comprehension). Patients with acute-onset aphasia, especially Broca’s or global aphasia, are often unable to speak for the first few hours or days. However, a wide variety of other insults can produce total cessation of verbal output (articulation and voice). The full differential diagnosis of mutism includes (a) psychiatric etiologies (schizophrenia, depression, catatonia, and psychogenic illness); (b) abulia/akinetic mutism (bilateral prefrontal, diencephalic, and midbrain lesions); (c) acute dominant supplementary motor area lesions; (d) pseudobulbar palsy; (e) locked-in syndrome from bilateral ventral pontine or midbrain lesions; (f) acute bilateral cerebellar lesions; (g) lower motor neuron lesions; and (h) laryngeal disorders.
C. Thought disorders. When an intact language apparatus is placed in service of an underlying thought disorder, bizarre utterances arise that superficially resemble the fluent aphasic output of patients with Wernicke’s or conduction aphasia. Demographic features are helpful, recognizing that schizophrenia with psychotic speech of new onset tends to appear in individuals in their 20s and 30s, whereas fluent aphasias cluster in older individuals with vascular risk factors. Several features of the utterances also distinguish thought-disordered from fluent aphasic speech. (a) Paraphasias are common in aphasia but rare in schizophrenia. (b) The neologisms of aphasics are frequent and changing, whereas those of schizophrenics are infrequent and consistent. (c) Open-ended questions tend to prompt briefer responses in aphasics than in schizophrenics. (d) Bizarre and delusional themes appear only in schizophrenic discourse.
COURSE
Both undamaged language or perilesional regions of the left hemisphere, and language homologous regions of the right hemisphere or both, are thought to support post aphasia recovery. Some degree of spontaneous recovery of language function is invariable after a static brain injury. An initial accelerated period of improved function occurs over the first few days or weeks after insult and is attributable to resolution of edema, ischemic penumbra, and other causes of dysfunction at a distance from the site of permanent injury. The second, slower phase of recovery reflects utilization of parallel circuits, retraining, and structural neural plasticity. The bulk of this functional recovery takes place in the first 3 months after injury, and some may continue up to 1 year, rarely longer. Among the aphasia syndromes, the greatest recovery compared with baseline tends to occur in Broca’s and conduction aphasias. Anomic aphasia is a common end stage into which other aphasia subtypes tend to evolve.
Factors favoring greater spontaneous improvement, as well as response to speech therapy, are young age, left-handedness or ambidexterity, higher education, smaller lesion size, no or few nonlanguage cognitive defects, absence of emotional difficulties such as depression and neglect, and strong family support. Patients with traumatic aphasia tend to recover more fully than patients with ischemic lesions.
REFERRAL
A. Neurologist. Most patients with aphasia should undergo neurologic consultation. The neurology specialist will confirm the presence of aphasia, clarify the type, aid in etiologic diagnosis, and provide the patient and family with an informed prognosis.
In selected cases, neurologists or physiatrists may consider pharmacotherapy of aphasia. Small case series have suggested that stimulants, cholinesterase inhibitors, dopamine agonists, and other neurotransmitter modulators may augment language therapy. Piracetam, donepezil, memantine, and galantamine have been found to be effective adjuncts to treatment of chronic poststroke aphasia in some studies. However, few well-validated randomized trials have been completed in this area, and more research is needed. Repetitive transcranial magnetic stimulation (rTMS) especially when combined with language therapy or other therapeutic approaches may promote language recovery, by releasing the perilesional fields from tonic inhibition.
B. Speech and language pathologist. All patients with aphasia should have an evaluation by a speech and language pathologist. The speech therapist will perform a formal diagnostic assessment, profiling the patient’s language strengths and weaknesses with normed tests. A variety of standardized language assessment batteries, including the Boston Diagnostic Aphasia Examination, the Western Aphasia Battery, the Porch Index of Communicative Ability, and the Communication Abilities in Daily Living, may be drawn on to survey a patient’s abilities. The therapist then employs the results to design and implement an individualized treatment program of aphasia therapy.
Systematic language rehabilitation programs improve patient outcome. Treatment is tailored to each individual’s pattern of linguistic and cognitive competencies and deficits, exploiting spared brain systems to reestablish, circumvent, or compensate for lost language capacities. A variety of deficit-specific programs are available to supplement general language stimulation. For nonfluency, treatments include (a) melodic intonation therapy, (b) sign language and other gestural communication training, and (c) communication boards. Syntax training may benefit agrammatism. Specific word-retrieval therapies have been developed for anomia and comprehension training programs for auditory comprehension deficits.
Speech therapy programs generally last for 2 to 3 months, in 30- to 60-minute sessions conducted two to five times per week. Recent studies suggest that intense treatment (at least 2 hours a day, at least 4 days per week) during a short period may be more effective than a similar number of sessions spread out over a longer time period. Self- and family-administered home exercises provide additional stimulation. Computer-based training is expanding in scope and sophistication.
C. Neuropsychologist. Patients who have major nonlinguistic cognitive deficits in addition to aphasia, and whose diagnosis is unclear, should undergo neuropsychological evaluation. Formal neuropsychological evaluation with tests that minimize language requirements allow a more detailed profiling of memory, visuospatial reasoning, executive function, praxis, and concept formation than can be obtained by bedside mental status examination. Findings may aid the physician in making a diagnosis by suggesting the pattern of neural system involvement and the speech pathologist in prescribing therapy by identifying the extent to which different extralinguistic capacities can support various compensatory strategies.
D. Patient support groups. The National Aphasia Association (National Aphasia Association, 350 Seventh Avenue, Suite 902, New York, NY 10001, 1-800-922-4622, www.aphasia.org) is an excellent resource for patients and their families. The American Heart/Stroke Association and National Stroke Association also provide beneficial programs and information.
Key Points
• The left cerebral hemisphere is dominant for language in over 90% of all individuals.
• Assessing for aphasia involves evaluation of fluency, repetition, comprehension, reading, and writing.
• Aphasia after stroke affects approximately one out of five stroke patients.
• Aphasia has a negative impact on health-related quality of life.
• Aphasia is complicated by high rates of depression and social isolation.
• Alexia without agraphia is often due to a lesion in the left occipital lobe extending to the posterior corpus callosum disconnecting the right visual cortex from language areas in the left temporal lobe.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








