Figure 46.5. Pathological findings in a 37-year-old patient with carotid artery dissection. The micrograph of the extracranial portion of the right internal carotid artery (panels A, B and C) shows a dissection between the outer layer and the tunica media causing stenosis of the arterial lumen (L). Intramural hemorrhage (asterisk) extends almost completely around the artery (panel A) (van Gieson stain, 4x). At higher magnification, the internal carotid artery shows fragmentation of the elastic lamina (panel b) (van Gieson stain, 25x) with accumulation of a pale substance in the tunica media shown by the blue staining of mucopolysaccharides (panel C) (Alcian Blue, x25). These changes are consistent with the diagnosis of cystic necrosis of the tunica media [Schievink, 2001].
In other cases, the hematoma originates in the tunica media and subsequently extends into the intima (subintimal dissection), allowing the passage of clots into the bloodstream. The hematoma may also expand into the adventitia (subadventitial dissection), weakening the vessel wall and allowing the development of pseudoaneurysms or dissecting aneurysms. The intracranial vessels are particularly vulnerable to this complication because of the absence of external elastic lamina and the thin adventitia. Once formed, the hematoma expands longitudinally in a proximal and distal way. Compression on the true lumen decreases blood flow, with the consequent risk of cerebral hypoperfusion. While subintimal dissections are associated with ischemic stroke, subadventitial dissections are associated with subarachnoid hemorrhage (SAH). Imaging studies obtained by nuclear magnetic resonance suggests that the pathophysiological mechanism that causes ischemia is, in most cases, thromboembolism.
It has been postulated that arterial dissection is a result of structural instability of the vessel wall. About 15-20% of patients with this disease have fibromuscular dysplasia, and 1-5% have hereditary connective tissue diseases such as type IV Ehlers-Danlos syndrome, Marfan syndrome, autosomal dominant polycystic kidney disease, osteogenesis imperfecta type 1, pseudoxanthoma elasticum, and α1 antitrypsin deficiency. In addition, arterial dissection has been linked to other arterial diseases such as cystic necrosis of the lamina media and Moyamoya disease. Other risk factors are migraine, recent infection, pregnancy, hyperhomocysteinemia, smoking, hypertension, and use of oral contraceptives.
The clinical presentation is variable but is most often accompanied by ipsilateral or widespread neck pain, facial pain or headache which may precede the occurrence of stroke for days or weeks. Subintimal dissections usually cause transient ischemic attack or stroke; the clinical presentation depends on the infarcted area but most patients present with amaurosis fugax, hemiparesis, or hemiparesthesia. Vascular distension is frequently observed in subadventitial dissections and can cause ispilateral Horner syndrome, pulsatile tinnitus or tinnitus, or cranial neuropathy (most often involving cranial nerves IX-XII). Vertebral dissections usually present with pain located in the neck, occipital region, or trapezius muscle; the pain can also radiate down the arm simulating a radicular neuralgia. The neurological deficits include diplopia, dysarthria, ataxia, and dizziness. The most common presentation is Wallenberg syndrome, which is caused by ischemia in the posterior inferior cerebellar artery territory. Intracranial dissections may present with SAH or ischemia. The latter is rarely transient and evolves into infarction more often than in extracranial dissections.
The diagnosis of arterial dissection has historically been based on radiographic findings obtained from digital cerebral angiography. In the classic form, dissection of the internal carotid artery shows a reduction in vessel calibre, sometimes with total occlusion, with its tapered distal end resulting in a typical “flame” image, followed by a long narrow column of contrast agent which may extend to the skull base (Figure 46.6).
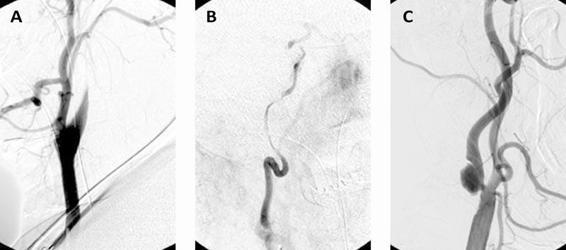
Figure 46.6. Conventional catheter angiography showing carotid dissection with reduction of the calibre of the internal carotid artery with its distal end forming a “scarf” (A), followed by a long, thin column of contrast material forming the “string sign” (B). A pseudoaneurysm caused by subadventitial dissection (C).
Other typical angiographic findings are arterial dilation in the proximal or distal to the dissection, double lumen, intramural or intraluminal clot formation, and dissecting aneurysm. intraluminal clots and dissecting aneurysm. Magnetic resonance imaging (MRI) and nuclear magnetic resonance angiography (MRA) are non-invasive methods that allow simultaneous evaluation of the major cervico-cerebral vessels and brain parenchyma. MRA reveals the presence of vascular stenosis or occlusion. The T1-fat-saturated sequence shows an expansion of the vascular wall and the presence of intramural hematoma, the latter visualized as a hyperintense lesion with a half-moon shape (Figure 46.7). The intensity of the hematoma on T1- and T2-weighted sequences depends on the age of the dissection. In addition, diffusion-weighted imaging, T2-weighted and fluid-attenuated inversion-recovery (FLAIR) sequences can identify parenchymal ischemic lesions. In comparison, vertebral artery dissections most commonly affect the poserioinferior cerebellar artery territory. The sensitivity and specificity of MRI to detect dissection of the extracranial portion of the internal carotid artery is 95% and 99%, respectively, whereas in the portion of the extracranial vertebral artery, the sensitivity and specificity of MRI are 60% and 98%, respectively. Computed tomography angiography (CTA) is a non-invasive method that allows the identification of double lumen (Figure 46.7).
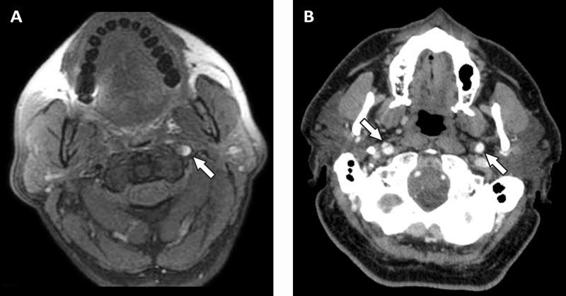
Figure 46.7. MRI T1 sequence scan with fat saturation showing dissection of the left internal carotid artery with a hyperintense lesion in the wall corresponding to an intramural hematoma (arrow, panel A). CT image showing a double lumen right internal carotid artery (arrow with solid line, panel B) and the normal left internal carotid artery (arrow with dotted line).
Because it is faster than MRI, CTA has become the radiographic test of choice for evaluating unstable patients. Recent studies suggest that CTA is superior to MRA for detecting vertebral artery dissections, and both techniques are similar for studying the carotid artery. However, MRI allows more detailed assessment of brain parenchyma, thus providing the physician with information of prognostic value. Finally, Doppler ultrasound of the carotid and transcranial Doppler ultrasound are useful in evaluating patients with suspected arterial dissection. The decrease or absence of blood flow in the affected vessel, distal retrograde flow and bidirectional flow in the carotid artery are indirect signs of high degree stenosis or obstruction in the appropriate clinical context and are suggestive of a diagnosis of dissection. With color Doppler ultrasound the intimal flap and double lumen can be observed (Figure 46.8); usually the flow velocity in the false lumen is lower than that in the true lumen; alternatively, the flow in both compartments may have opposite directions.
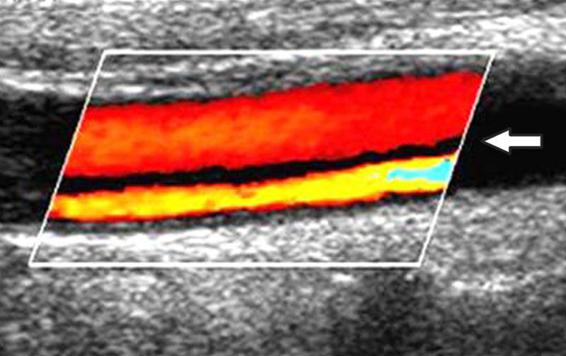
Figure 46.8. Doppler ultrasound image of the carotid artery in a patient with arterial dissection. The image shows a double lumen, a high flow rate (lower) and a low flow rate (higher), separated by an “intimal flap” (white arrow).
The treatment of ischemic stroke associated with arterial dissection is controversial. Currently, there are no randomized trials comparing the efficacy of antiplatelet agents and anticoagulants in the management of this disease. The treatment of intracranial and extracranial dissection differs according to the authors. On the basis of the pathophysiological mechanism of ischemia in patients with extracranial dissections, the use of intravenous heparin is usually recommended, followed by oral anticoagulants for 3-6 months, with the aim of achieving an international normalized ratio (INR) between 2 and 3. More controversial is the use of anticoagulants in patients with intracranial dissection because of the risk (probably low) of SAH in patients with subadventitial dissection. The use of anticoagulants is related to the theoretical risk of intramural hematoma expansion, but the low number of reports describing this complication suggests that it is unusual.
If the use of anticoagulants is contraindicated, we suggest the use of antiplatelet agents that can be continued indefinitely. The use of thrombolytic agents in patients presenting within 3 hours of symptom onset has been described. Most of these reports include a small number of patients. Based on the mechanism underlying the ischemia, it is likely that this treatment is effective in patients who present in the hyperacute state. However, no prospective studies investigating the efficacy and adverse effects of thrombolytics in the treatment of dissection pressure are available. Finally, a few studies describe the use of angioplasty and stenting as alternatives in the management of patients with intra-or extracranial dissections. Manipulation of the dissected vessel may cause blood clots to shed, with the risk of causing further ischemia. Therefore, this treatment is reserved for cases refractory to medical treatment with anticoagulants.
The risk of recurrence of this disease is low. In a retrospective study involving 459 individuals with carotid dissection, the risk of recurrent ischemia at 31 months was 0.9%; half of the cases occurred in the subacute period and the other half was due to recurrent dissection.
46.5 Thrombophilia
It has been estimated that about 5-10% of cases of cerebral ischemia in the general population are caused by thrombophilia; this percentage is even higher in younger patients. Maintenance of homeostasis depends on the combined action of procoagulant and anticoagulant mechanisms, and the deficiency of inhibitory factors as well as the elevated levels of certain mediators of the coagulation system increase the risk of thrombosis. There are numerous inherited and acquired hypercoagulable states, those most frequently encountered in clinical practice are summarized in Table 46.3.
Hereditary |
|
Acquired |
|
Table 46.3. Hypercoagulable states inherited and acquired more frequently encountered in clinical practice.
Patients with inherited thrombophilias typically present with venous thrombosis; arterial thrombosis, however, is observed less frequently. Thus, the pathophysiological mechanisms of cerebral ischemia more common in patients with thrombophilia are venous sinus thrombosis and paradoxical embolism of thrombus originating in the veins of the legs or pelvis. The prevalence of inherited hypercoagulable states in Caucasians is low, and the role of these as causative agents of ischemia is controversial (Table 46.4).
Syndrome | Control group asymptomatic (%) | Patients with deep vein thrombosis (%) | Patients with a family history of venous thrombosis (%) | Patients with ischemic stroke | |
% | OR (95% CI) | ||||
PC deficiency | 0.14-0.5 | 3.2 | 4.9 | 1.4 | 0.7 (0.2-3.1) |
PS deficiency | 0.1 | 2.2 | 5.1 | 0.9 | 0.9 (0.1-6.7) |
ATIII deficiency | 0.02-0.2 | 1.1 | 4.2 | 5.2 | 1.3 (0.5-3.3) |
FVL | 3-6 | 19 | 46 | 4.6 | 2.1 (0.6-6.8) |
MGP | 1-2 | 6.3 | 18 | 3.7 | 1.9 (0.5-6.2) |
Table 46.4. Prevalence of hereditary hypercoagulablility syndromes in Caucasians.
ATIII = antithrombin III; CI = confidence interval; FVL = factor V Leiden; MGP = 20210A mutation of the prothrombin gene; OR = odds ratio; PS = protein S.
A meta-analysis including a total of almost 18,000 cases and 58,000 controls was used to study the role of Factor V Leiden (FVL), the mutation 20210 of the prothrombin gene (PGM), and polymorphism in the gene for the enzyme methylenetetrahydrofolate reductase (MTHFR) in the development of cerebral ischemia. The risk of stroke was 1.33 (95% confidence interval [CI], 1.12 to 1.58) in patients with FVL, 1.24 (95% CI, 1.08 to 1.42) in patients with MTHFR, and 1.44 (95% CI, 1.11 to 1.86) in patients with PGM. In a meta-analysis including approximately 54,000 subjects studied the association between inherited thrombophilias and arterial ischemia (including myocardial infarction, ischemic stroke and peripheral vascular disease). The risk of arterial ischemia in patients under 55 years with FVL was 1.37 (95% CI, 0.96 to 1.97), 1.66 (CI 95%, 1,13-2, 46) in patients with MTHFR and 1.41 (95% CI, 1.13 to 1.76) in patients with PGM.
Antiphospholipid antibodies are circulating IgG, IgM or IgA antibodies that recognize anionic and neutral membrane phospholipids. From a clinical point of view, the most relevant are the lupus anticoagulant, anticardiolipin antibodies and β2-glycoprotein antibodies. The phosphatidylserine antibody is seen less frequently and has been less studied. Antiphospholipid antibodies are mostly acquired, although they can sometimes be inherited. Antiphospholipid syndrome (APS) is characterized by the presence of antiphospholipid antibodies in the context of arterial or venous thrombosis. The prevalence of APS in the general population is estimated to be between 1.0% and 6.5% and its presence is suspected in individuals with history of repeated spontaneous abortions, connective tissue diseases (such as lupus), the presence of livedo reticularis on clinical examination, and ischemia of unknown origin in young patients. In laboratory studies, APS is suggested by a prolonged partial thromboplastin time, a false positive venereal disease research laboratory (VDRL) test, and a moderate thrombocytopenia. Other findings that suggest the presence of this condition are summarized in Table 46.5.
Clinical examination |
|
Laboratory tests |
|
Table 46.5. Clinical and laboratory features associated with antiphospholipid syndrome.
APS produces a hypercoagulable state and is observed in approximately 10% of patients with cerebral ischemia. This percentage is even higher in younger patients. The IgG isotype was statistically associated with the occurrence of stroke, and IgM isotype is considered an acute phase reactant whose level increases after certain infections. The role of IgM and IgA in ischemic stroke has not been clarified yet.
The management of venous or arterial thrombosis in patients with hypercoagulablity states is controversial. The presence of deep vein thrombosis (DVT) in the legs, and probably in the pelvis, in individuals with ischemic stroke and inherited thrombophilia, should prompt the assessment for cardiac variants causing right-to-left shunt and paradoxical embolism. If there is no alternative mechanism of ischemia, the use of heparin followed by warfarin sodium is recommended in order to achieve an INR between 2 and 3.
In the WARSS APASS trial (Antiphospholipid Antibodies and Stroke Study – Warfarin Aspirin Recurrent Stroke Study), the effectiveness of warfarin compared to aspirin in preventing the recurrence of cerebral ischemia in individuals with antiphospholipid antibodies was compared. This study included a total of 1770 individuals, 720 had antiphospholipid antibodies and 1050 were controls. The risk of recurrent stroke at 2 years was similar in both groups. Moreover, the frequency of recurrent cerebral ischemia in the individuals with antiphospholipid antibodies was similar. On the basis of these results, there is no evidence to justify the use of oral anticoagulants in patients with first ischemic stroke and antiphospholipid antibodies; in such patients the use of antiplatelet agents is reasonable. Differently, in patient with cerebral ischemia and APS we recommend the use of heparin followed by warfarin sodium in order to achieve an INR between 2 and 3. Due to the autoimmune mechanism underlying the APS, the possible role of iatrogenic immunosuppression with steroids or immunosuppressive drugs such as azathioprine, cyclophosphamide, cyclosporine, intravenous gamma globulin, and plasmapheresis has been suggested. The benefits and potential adverse effects associated with the use of these agents in preventing recurrent ischemic stroke have not been evaluated in clinical trials; therefore, these treatments are considered experimental and reserved for patients refractory to antithrombotic therapy.
46.6 Sickle Cell Anemia
Sickle cell anemia (SCA) is an autosomal recessive genetic disorder caused by a mutation in codon 6 of the β-globin gene. This mutation causes the substitution of a residue of valine for glutamic acid which induces a change in the spatial structure of the hemoglobin molecule (HbS). Two of the most serious complications of this disease are ischemic stroke (75% of cases) and cerebral hemorrhage (25% of cases). The most frequently affected population is children and young adults. Under low oxygen tension, deoxygenated HbS forms insoluble polymers that precipitate in the microvessels. The endothelial injury that occurs in this condition generates a non-inflammatory arteriopathy affecting the large vessels of the circle of Willis, causing stenosis and ischemia. In areas of severe stenosis, a phenomenon of neovascularization may occur that generates small collateral vessels (moyamoya), which are fragile and increase the risk of cerebral hemorrhage. Other pathophysiological mechanisms of ischemia that can occur in this disease include hypercoagulability, related to an increased production of thrombin, platelet activation, and high levels of inflammatory mediators, and cardioembolic stroke. The latter, however, is rarely observed.
In the early 1990s, Adams showed that the average flow velocity in the middle cerebral artery of patients with SCA as measured by transcranial Doppler ultrasound (TCD) is directly correlated with the risk of stroke. This observation was the starting point for the Stroke Prevention Trial in Sickle Cell Anemia I (STOP I) study. This trial compared medical treatment with regular transfusion in the prevention of cerebral ischemia in patients with SCA. Approximately 2000 individuals with SCA (age range, 2-16 years) were studied by TCD and those with flow velocity in middle cerebral artery >200 cm/sec were randomized to receive medical treatment or periodic transfusions to reduce the level HbS <30%. The annual risk of stroke was 10% in the control group and 0.5-1% in those treated with regular transfusions. Moreover, in the latter group a significant decrease in flow velocity in the middle cerebral artery was observed. This trial was followed by the STOP II trial which investigated whether it was safe to stop periodic transfusion in patients who had normalization of flow velocity in the middle cerebral artery. This study was aborted early after seeing a rapid reversal of the hemodynamic benefit achieved and the occurrence of two cases of cerebral ischemia after regular transfusions were discontinued.
The management of young adult patients with SCA and stroke include the use of antiplatelet agents and the control of classical cardiovascular risk factors associated with the occurrence of cerebral ischemia. The use of regular transfusions can be considered in order to reduce the HbS <30-50%. However, it should be noted that most of the patients analyzed in STOP I and II studies were children and it is unclear whether the results can be extrapolated to adults. In addition, chronic transfusion is associated with complications and carries the risk of causing iron overload which requires the use of oral chelators.
46.7 Patent Foramen Ovale
Based on autopsy studies, it was estimated that the prevalence of patent foramen ovale (PFO) in the general population is 26%. Its pathological value is controversial. The PFO, as well as atrial septal defects, allows communication between venous and arterial blood and increases the risk of paradoxical embolism. Right-to-left shunt may be revealed or increased by manoeuvres or activities that increase intrathoracic pressure, the flow direction also can be reversed in certain pathological conditions.
In a clinical trial in patients <55 years of age, the prevalence of PFO was 40% in those with ischemic stroke and 10% in the control group (P <0.001). Within the group of patients with ischemia, the prevalence of PFO in individuals with known cause of stroke was half of those without an identifiable cause of stroke (20% versus 40%, P <0.10). This study, like others, shows an association between PFO and the presence of ischemia, particularly in individuals with cryptogenic stroke. In addition, several studies show that the association of this disease with ischemic stroke increases with the size of PFO. On the basis of observations it has been suggested that, besides allowing paradoxical embolism, PFO may be a direct causal agent of cerebral ischemia.
There are several techniques that allow the diagnosis of PFO. Among the echocardiographic techniques, transthoracic echocardiography (TTE) is the most widely used method. Trans-oesophageal echocardiography (TEE) is more invasive, but its sensitivity is higher and it is considered the reference method. Atrial septal defect is a discontinuity in the interventricular septum, whereas PFO has a valve-like structure. In addition, the PFO does not always manifest at rest, requiring in such cases the use of techniques that can provoke it, as the Valsalva manoeuvre. In transcranial Doppler, micro-bubbles are used as a contrast method; they are injected intravenously while the peripheral middle cerebral artery is interrogated. The presence of microbubbles in the arterial system in a “shower” or “curtain” pattern is consistent with a right-to-left shunt. Depending on the clinical context, it can suggest the presence of PFO or arteriovenous fistula. In comparison with TEE, transcranial Doppler has a sensitivity in the detection of PFO of 68-89% and a specificity of 92-100%.
The management of ischemic stroke of unknown cause in patients with PFO alone (without atrial septal aneurysm) has generated controversy. There is no evidence in the literature to suggest that warfarin is superior to antiplatelet agents in preventing recurrent stroke. As an alternative to medical treatment, surgical or percutaneous closure of PFO has been suggested. In a recent prospective study, however, PFO closure in patients with cryptogenic stroke or TIA did not offer a greater benefit compared to medical therapy alone for the prevention of recurrent ischemic events. At present, medical treatment with antiplatelet agents for patients with cryptogenic stroke and PFO is recommended. Treatment with warfarin is reserved for individuals at high risk of recurrence, as those with hypercoagulable states or DVT. Percutaneous closure of PFO is recommended only for recurrent stroke cases unresponsive to medical treatment.
46.8 Retinococleocerebral Artery (Susac’s Syndrome)
Susac’s syndrome (SS) is characterized clinically by the triad of encephalopathy, fluctuating sensorineural hypoacusia and sudden visual loss. The pathophysiological mechanism is a microangiopathy that affects the vessels of the brain, retina and cochlea producing microinfarcts. The cause of this disease is unknown, although there is evidence suggesting a mechanism of autoimmunity whose target organ is the endothelial cell. Histopathology shows the presence of a predominantly lymphocytic perivascular infiltrate, microinfarcts, adventitial thickening, and endothelial proliferation (Figure 46.9).
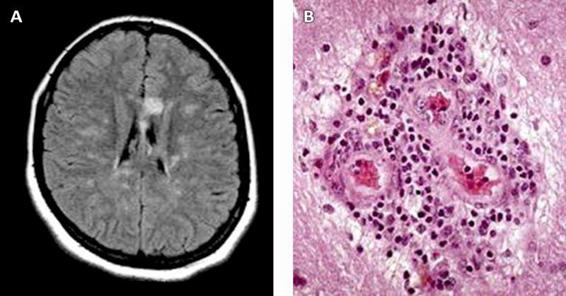
Figure 46.9. A Magnetic resonance imaging (fluid attenuated inversion recovery [FLAIR] sequence) axial section of a patient with Susac’s syndrome showing an ischemic lesion in the form of a “snowball” in the ischemic knee of the corpus callosum. Note the smaller ischemic lesions affecting mainly the white matter. B. Brain biopsy preparation showing adventitial thickening, endothelial proliferation and a predominantly periarteriolar lymphocytic infiltrate (staining: hematoxylin and eosin, 400x) [Fox, 2006].
Magnetic resonance imaging of the brain shows the presence of microinfarcts with a typical “snowball” appearance located, more often, in the corpus callosum and the supratentorial white matter (Figure 46.9).
The diagnosis of SS is mainly clinical and radiological. Encephalopathy and the motor or sensory deficits have been observed in 80% of cases, vision loss and hearing loss have a frequency of 46% and 52%, respectively. The typical triad is much less common, occurring only in 20% of individuals. In the peripheral blood there is usually an elevation of serum markers of inflammation such as erythrocyte sedimentation rate and C-reactive protein, and the cerebrospinal fluid shows elevated protein and lymphocytic pleocytosis. The ophthalmologic exam is characterized by occlusion of branches of the central retinal artery, and the audiogram shows hearing loss with a predilection for mid and low frequencies.
Treatment of SS is controversial and is based largely on studies with a small number of patients and the postulated mechanism of autoimmunity. Intravenous steroids are often used in the acute state. In refractory cases, plasmapheresis or intravenous gamma globulin administration are suggested. The use of other immunosuppressive drugs like azathioprine and cyclophosphamide has been reported in the literature, with variable results. Antiplatelet and anticoagulant agents are usually ineffective for treating this condition.
Susac’s syndrome has a self-limiting course lasting from a few months to 5 years. Early diagnosis and rapid initiation of immunosuppressive therapy have a direct impact on the prognosis of these patients.
46.9 Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy (CADASIL)
CADASIL is an autosomal dominant systemic arteriopathy caused by a mutation ni the Notch3 gene. This gene is located on chromosome 19p 13.2-13.1 and encodes a membrane receptor involved in signal transduction mechanisms and neural development of Drosophila. In humans, its role is unknown, but it has been postulated that it’s important for maintaining the structural stability of vascular smooth muscle cells. In patients with CADASIL, the membrane receptor Notch3 accumulates in the plasma membrane of these cells in the intracranial and extracranial vessels; electron microscopy shows intraneuronal granular osmiophilic deposits. Clinically, CADASIL is associated with migraine (usually with aura) that develops in 40-70% of cases in the third or fourth decade of life. The most severe complication of this entity is recurrent ischemic stroke which occurs in 80-85% of patients 30-50 years of age. Other typical clinical manifestations are psychiatric disorders and subcortical dementia.
Magnetic resonance imaging of brain shows ischemic lesions in the periventricular white matter and around the basal ganglia, with particular focus on the anterior pole of the temporal lobe and external capsule. These changes are not specific to CADASIL and may be absent in initial stages of the disease. The diagnosis of CADASIL is based on the identification of the Notch3 gene mutation. Electron microscopy of skin biopsy shows the presence of granular osmiophilic deposits adjacent to the basement membrane of smooth muscle cells of the arteriolar wall (Figure 46.10). These findings have a sensitivity of 45% and a specificity of 100% in the diagnosis of CADASIL; the immunological staining using anti-Notch3 monoclonal antibody increases the sensitivity of skin biopsy to 100%. Finally, this disease can also be diagnosed by identifying the mutation in the Notch3 gene in a sample of peripheral blood. This method, however, is expensive and laborious.
In the absence of specific treatment for this disease, efforts should focus on identifying and correcting cardiovascular risk factors. Death usually occurs 20 years after the onset of symptoms.
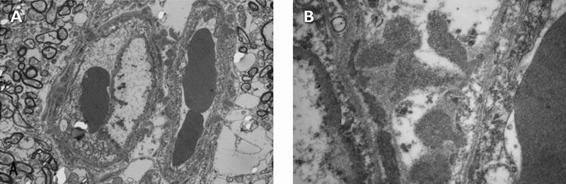
Figure 46.10. Electron microscopy of skin biopsy preparation from a patient with CADASIL showing arteriolar ultrastructure. Panel A: granular osmeophilic deposits are seen in smooth muscle cells. At higher magnification, these granular osmeophilic deposits are irregularly shaped, 0.2 to 1 mm in size and consist of fine granules [Arima, 2003].
46.10 Fabry Disease
Fabry disease (FD) is a genetic disorder caused by deficiency of-galactosidase α (α-gal). This enzyme is a lysosomal hydrolase involved in the metabolism of glycosphingolipids. Its deficiency causes the intralisosomal accumulation of sphingolipids with α-galactosyl residues, particularly globotriaosylceramide (Gb3). The gene coding for the α-gal is located on the X chromosome and more than 400 mutations have been described. These are de novo mutation; therefore, the lack of family history of FD does not exclude the diagnosis of this condition. The age of onset is variable and depends on the residual enzyme activity. The most frequent manifestations of FD are listed in Table 46.6. Historically, it was considered that FD affected men and women were asymptomatic carriers. However, there are numerous reports indicating that, due to non-random tissue-specific X chromosome inactivation, women may have atypical presentations.
Dermatologic | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



