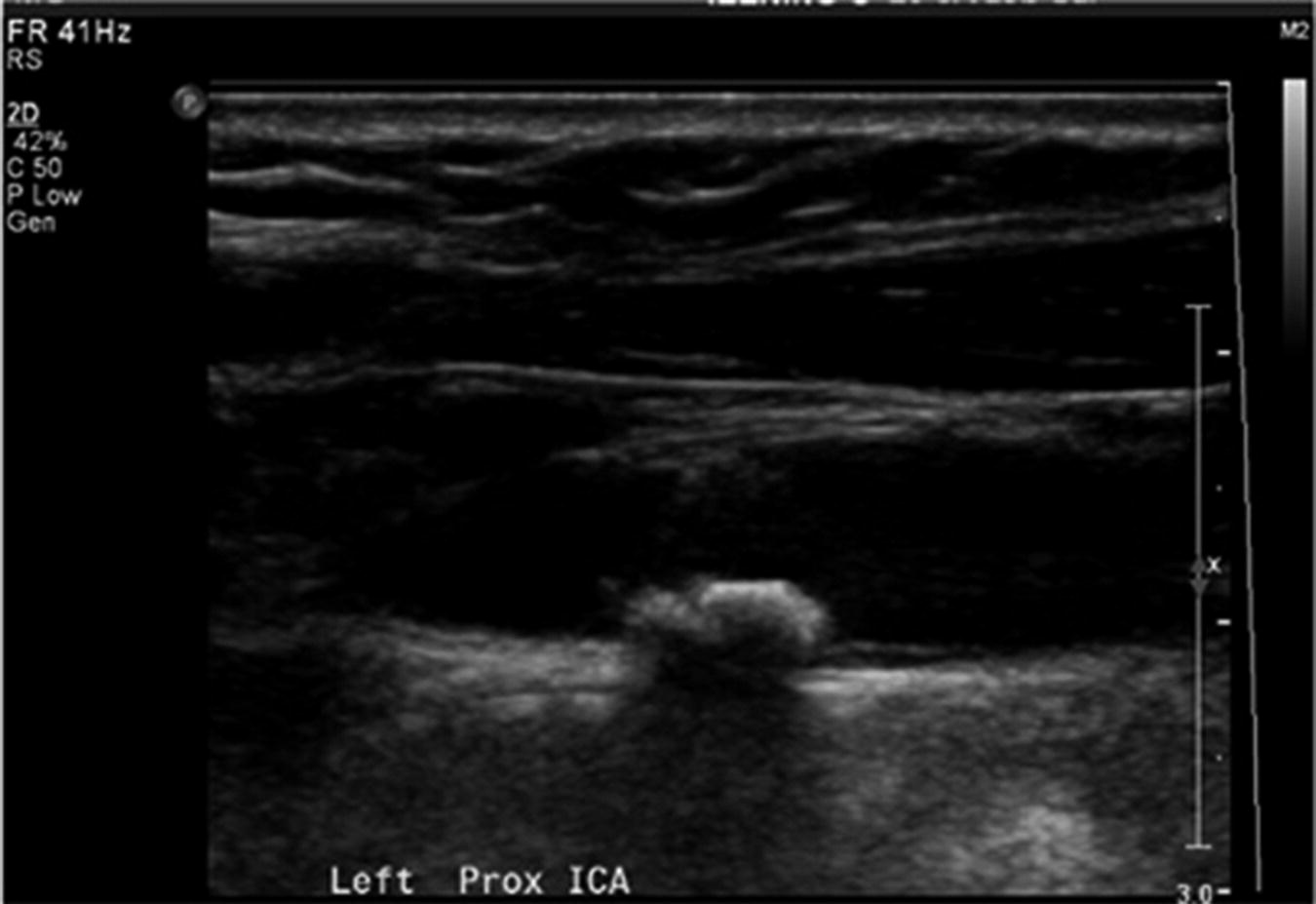
Duplex Doppler image of the carotid bifurcation
Information gleaned from preoperative ultrasound
Anatomical factors • Configuration of carotid vessels • Level of carotid bifurcation • Tortuosity of ICA |
Plaque factors • Degree of stenosis • Plaque type |
Physiological factors • Flow velocities • Integrity of circle of Willis – Contralateral ICA – Vertebral arteries |
Duplex ultrasound criteria for defining stenosis
% Stenosis NASCET | Peak systolic velocity (PSV, cm/s) | PSVICA/PSVCCA ratio |
|---|---|---|
<50% | <125 | <2 |
50–69% | ≥125 | 2–4 |
60–69% | ||
70–79% | ≥230 | ≥4 |
80–89% | ||
>90% | ≥400 | ≥5 |
Near occlusion | High, low-string flow | Variable |
Occlusion | No flow | Not applicable |
Limitations common to all ultrasound assessments for carotid artery disease include anatomical factors such as a short neck and/or a high carotid bifurcation that obscures the carotid bifurcation deep to the mandible and plaque factors such as highly calcified plaques that result in acoustic shadowing [9]. In this context, an additional imaging modality is indicated.
4 Developments in Ultrasound Technology
At the present time, the percentage diameter reduction in the ICA is used as the main predictor for stroke risk. New research into the mechanisms of plaque rupture and atheroembolic stroke suggests that the degree of narrowing is an imperfect predictor of stroke risk. It is often observed that many patients with high-grade carotid stenosis remain asymptomatic for many years, while others with moderate stenosis develop neurologic symptoms sooner [10].
It has been proposed that other factors such as plaque composition and hemodynamic forces can play a role in determining the risk of stroke. Magnetic resonance image (MRI) studies of stroke patients with only mild carotid artery stenosis have shown that these plaques exhibit other features that suggest vulnerability [11]. Surrogate markers of plaque vulnerability include plaque volume, lipid necrotic core size, surface ulceration, intraplaque hemorrhage, and hemodynamic effects around the plaque. Advances in ultrasound technology have enabled interrogation of the factors that predispose to plaque vulnerability, permitting identification of plaques at high-risk of imminent embolization, potentially resulting in stroke.
Animal studies indicate that enlarging lipid cores, increasing intraplaque hemorrhage and thinning of the fibrous cap all predispose plaques to rupture; however these factors are on a continuum and, unlike flow velocity measurements, at the present time discrete thresholds do not exist that categorize the risk of stroke. Furthermore, one-time imaging does not provide an insight into the long-term biological evolution of a carotid plaque and application of new techniques would necessitate serial measurements alongside clinical correlation before these markers were validated for the stratification of plaque vulnerability [12].
5 Brightness Mode Imaging
Brightness mode (B-mode) imaging is a form of two-dimensional imaging that displays anatomic wall features. Different tissues reflect ultrasound waves to varying degrees, and this leads to structures that appear either heterogeneous, or relatively hypoechoic or hyperechoic. Gray-Weale devised a classification to describe the appearance of different carotid plaques and it was observed that plaques with hyperechoic signals were more likely to be found in patients with neurological symptoms [13]. This method, however, had two major limitations; that the classification depended on subjective visual estimation and that the technique for image acquisition also introduced considerable variability, leading to high levels of interobserver and intraobserver variation.
El-Barghouty et al. [14] introduced standardization for image acquisition and brightness using the blood column and carotid adventitia. Carotid plaques were outlined and the median brightness of pixels in a single longitudinal image of the plaque was measured and expressed as a gray-scale median (GSM) value.
One thousand two hundred and twenty-one patients with 50–99% carotid stenosis were followed for 6–96 months. GSM ≤ 40, longitudinal plaque area (hazard ratio = 1.92; 95% confidence interval, 1.50–2.46; P ≤ 0.001) and the size of discrete white areas in the plaque image (hazard ratio = 2.10; 95% confidence interval, 1.32–3.35; P ≤ 0.002) were all found to be strong predictors for future stroke [15].
Supporting the theory that rupture of the fibrous cap that overlies the lipid core of an atherosclerotic plaque leads to thromboembolic events, histological studies have corroborated that the necrotic core of unstable plaques is located closer to the lumen than in asymptomatic plaques [16].
Juxtaluminal black area (Reproduced from Kakkos et al., 2013 with permission from Elsevier) [17]
6 Pixel Distribution Analysis
Our current understanding of atherosclerosis purports that atherosclerotic plaques begin with deposition of lipid to form fatty streaks that gradually coalesce to form a lipid core. Over time, a fibroatheroma forms as fibrous tissue accumulates over the lipid core to form a fibrous cap.
Much of our understanding of atherosclerotic disease is influenced by study of the coronary arteries. When studying coronary artery disease, it emerged that the risk of coronary events was not simply determined by the degree of coronary artery stenosis. Indeed, not all coronary culprit lesions were high-grade stenoses. Emerging data suggests that atherosclerotic plaques are rendered unstable/vulnerable through an enlarging lipid core, intraplaque hemorrhage, fibrous cap thinning/rupture, and finally ulceration. These histological changes in plaque morphology have been observed in carotid endarterectomy specimens retrieved from patients with symptomatic (stroke/TIA) carotid artery disease [12, 18, 19].
The potential translational benefits of improved ultrasound carotid imaging are wide-ranging. In the modern era, when patients may be considered more or less suitable for either surgical carotid endarterectomy or endovascular carotid artery stenting, it is conceivable that identification of plaque stability may influence the choice of treatment modality. For instance, patients with large juxtaluminal necrotic cores may be more vulnerable to endovascular atheroembolization when disturbed by a guide wire, balloon or manipulation of a stent. Improved, noninvasive ultrasound imaging could therefore stratify risk of treatment in asymptomatic patients; it could also quantify plaque responses to medical management.
Detection of these segmental areas of plaque instability was traditionally not possible using Gray-Weale or GSM as they were missed. Ideal noninvasive morphological assessment would not only detect these areas of plaque instability but also elucidate their distribution using a technique with a low intraobserver and interobserver variability.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


