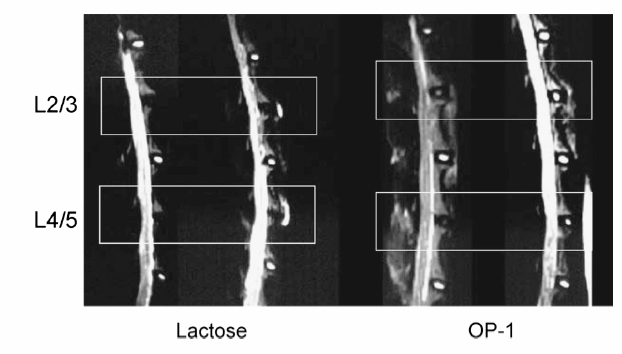
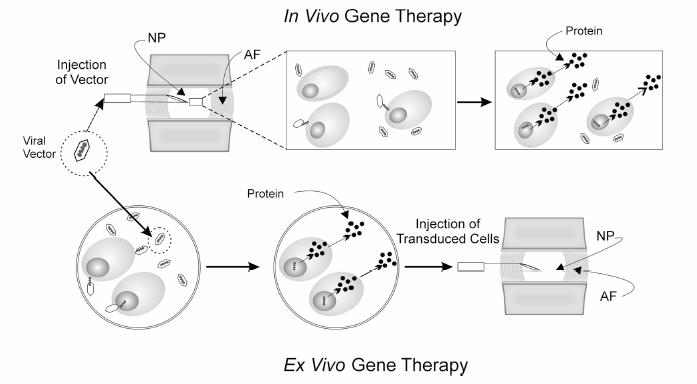
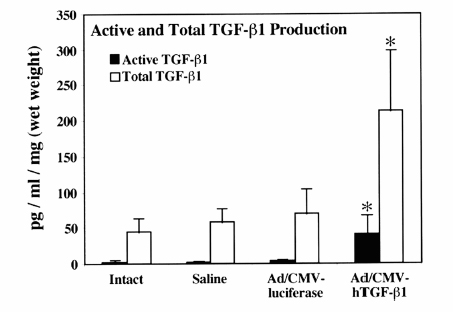
20 Biologic Therapies for Disc Repair Corey A. Pacek, Gwendolyn A. Sowa, Nam Vo, and James D. Kang The lumbar intervertebral disc (IVD) is the most common site of degenerative changes in the adult spine. Although early patient symptoms may sometimes be successfully managed through the use of nonoperative modalities such as medications, physical therapy, and interventional procedures, these treatments have not been shown to alter the natural history of degenerative disc disease (DDD). The final common pathway of these degenerative changes often leads to stenosis and subsequent compression of spinal nerve roots. Typical surgical treatment consists of correcting deformity, decompressing nerve roots, and achieving bony fusion for stability, when unstable. Although surgical treatment of the degenerative lumbar disc is relatively effective in these ways, these modalities are designed to treat the end stages of DDD. Biologic therapies, on the other hand, seek to treat earlier stages of DDD when the spinal architecture is less degenerated. Though surgical treatments are decompressive and reconstructive, many new avenues of biologic therapy for DDD are reparative in nature. DDD is commonly thought to be the result of an imbalance in the anabolic and catabolic processes within the disc, where the degradative processes overwhelm the synthetic capabilities of the disc. The theory behind many biologic therapies lies in restoring this imbalance back toward normal homeostasis by supporting the anabolic or inhibiting the catabolic pathways. Several basic approaches exist for achieving this goal. The most straightforward of these approaches is that of directly injecting therapeutic biomolecules within the IVD that will lead to an increase in anabolic activity. Building on this strategy, another approach consists of modifying the disc cells genetically such that synthetic activity remains increased for longer periods of time than can be achieved with a one-time therapeutic intervention. A final tactic involves the transplantation of modified cells to create lasting increases in the synthetic processes of the IVD. These strategies shall be addressed in turn as to their method and ongoing research into optimization of effectiveness. The most straightforward approach to biologic therapy for disc repair is the injection of factors that will result in an increase in the synthetic activity of the disc. After injection into the degenerated nucleus pulposus (NP), it is hypothesized that the stimulatory effect of the injected factor will slow or reverse the degenerative cascade. The appeal of this modality lies in its simplicity of institution. To achieve this goal, several anabolic factors—such as transforming growth factor β-1 (TGF-β1),1 bone morphogenic protein-2 (BMP-2),2 bone morphogenic protein-7 (BMP-7),3 and growth and differentiation factor-5 (GDF-5)4—have been identified as possible treatments for injection therapy. However, only BMP-7 and GDF-5 have been researched as to the utility of in vivo injections. Significant research has been done exploring the role of BMP-7, or osteogenic protein-1 (OP-1), as a possible therapy for DDD. Injection of BMP-7 into the IVD is hypothesized to stimulate the IVD cells to increase their production of proteoglycans. Increased proteoglycan content, in turn, is thought to increase the tonicity of the NP and thus increase the water content. It is hoped that through this process an IVD with the morphology and biochemistry closer to that of a normal IVD is produced. BMP-7 has been shown in vitro to stimulate the production of proteoglycans in both NP cells as well as anulus fibrosus (AF) cells.3 An et al5 reported an increase in disc height in the spines of healthy rabbits after BMP-7 in vivo intradiscal injections. Biochemical analysis following the sacrifice of these animals showed a significant increase in proteoglycan content as compared with control injections of lactose. Following this, Masuda et al6 performed intradiscal injections of BMP-7 into degenerative rabbit discs, using a stab model of disc degeneration to test the effect of BMP-7 on the degenerating IVD. The subsequent disc space narrowing that is often seen with DDD was restored in the injected discs, and this height was maintained throughout a 24-week period after injection (Fig. 20.1). These studies were the first to suggest that a single injection of a growth factor could lead to the repair of disc degeneration. GDF-5 is another member of the TGF-β superfamily and BMP subfamily that has shown some promise with respect to biologic therapy of the IVD. GDF-5 deficient mice have been shown to undergo accelerated disc degeneration.7 GDF-5 has been shown to increase proteoglycan content in both NP and AF cells as well as stimulate cell proliferation as assessed by DNA content analysis in vitro. A single injection of GDF-5 into rabbit NP also has been shown to restore the disc height of degenerated rabbit discs,4 presumably through the stimulation of proteoglycan synthesis and the reestablishment of water balance. Fig. 20.1 Degenerated lumbar discs (L2-L3 and L4-L5) were injected with either lactose or bone morphogenic protein-7 (BMP-7/OP-1). The signal intensity of the BMP-7 injected discs shows increased signal on T2-weighted magnetic resonance imaging as compared with the lactose control. (From Masuda K, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit annular puncture model. Spine 2006;31:742–754; reprinted with permission.) The injection of growth factors into the IVD has potential utility for biologic therapy for DDD. Though the effects of several growth factors on NP and AF cells have been studied, only BMP-7 and GDF-5 have been shown in an in vivo model to have promise for repair of degenerated disc tissue. Much more research is needed in this area, as are long-term studies to evaluate the ability for a single injection of these factors to maintain the beneficial changes that it appears to initiate. It is known that single, subcutaneous injections of growth factors have a half-life of approximately 20 minutes.8 Although injected factors would likely possess a much longer half-life in an avascular structure such as the IVD, it is not known whether multiple injections would be needed throughout the natural history of a patient with DDD. Because of this potential limitation, avenues into other biologic therapies are currently being evaluated as well. A second strategy of biologic therapy for IVD repair is that of gene therapy. The main goal of gene therapy is to transfer a gene of interest to the cells of the IVD with the subsequent expression of that gene in large amounts, restoring or upregulating production of a beneficial gene product (Fig. 20.2). With efficient and lasting transduction of the NP cells of the disc, a constant supply of the therapeutic protein would be available to the cells instead of that which would be available from a one-time injection. It is this feature that makes gene therapy an attractive treatment modality. Transduction can take place in vivo through the direct injection of a vector containing the gene of interest, or the cells can be first transduced in vitro and then injected into the IVD, termed ex vivo gene therapy. Both methods entail their own advantages and limitations, which will be highlighted following a brief discussion of recombinant vectors. A vector is responsible for the transfer of the target gene to the host cell. Two main categories of vectors exist: nonviral and viral. Nonviral vectors, such as liposomes, biolistics or “gene guns” using naked DNA, and DNA-ligand complexes, have not been commonly used for gene therapy for DDD due to their low efficiencies of gene transfer, difficulty of application, and problems with cell death and toxicity.9–11 Viral vectors commonly used for gene therapy include retroviruses, adenovirus, and adeno-associated virus. They function through the natural protein machinery of the virus to attach to cell membranes and inject DNA. However, the genes necessary for continued viral replication are absent; thus these recombinant viral vectors serve only to deliver the DNA to the host cell. Retroviral vectors, though highly efficient at infecting actively dividing cells, are a less optimal choice for gene therapy of DDD because the cells of the IVD are typically nondividing. Use of adenoviral vectors in gene therapy has shown excellent transduction efficiency. However, their use raises safety concerns due to their immunogenic nature. These limitations have led researchers to focus most recently on adeno-associated virus, a much less immunogenic virus than adenovirus. Although the use of adeno-associated virus lessens the risk of host reaction, much lower gene transfer efficiency is observed. Thus, the efficiency of gene transfer must be balanced with the potential immunogenicity of the viral particle. Fig. 20.2 Scheme of gene therapy. In vivo gene therapy involves the direct injection of vector into the nucleus pulposus (NP) of the disc, with subsequent NP cell transduction and protein production. Ex vivo gene therapy involves the transduction of cells in vitro and subsequent injection of cells into the NP of the disc. AF, anulus fibrosus. The potential benefit of in vivo gene therapy is that a single, noninvasive injection is hypothesized to result in consistent, long-term expression of specific growth factor and subsequent increase in extracellular matrix. This method, however, depends on the accuracy of the injection, as well as the efficiency of transduction from this single dose of vector. Additionally, this method requires the host to be exposed to a viral vector, which could lead to an immunogenic reaction. However, such a reaction is less likely in the IVD because it is a relatively avascular and immunogenically isolated organ. BMP-2 has been shown in the past to stimulate NP cells to increase their production of extracellular matrix proteins.2 Preliminary evidence by Shimer et al12 has shown the potential utility of BMP-2. Degeneration of the IVD as assessed by MRI was seen to be slowed significantly through the transduction of NP cells with adenovirus containing the gene for BMP-2. It was thus shown for the first time that gene therapy was able to alter the natural history of DDD. TGF-β1 has also been shown to increase IVD extracellular matrix production,1 making it an appropriate choice for gene therapy. Nishida et al13 injected adenovirus containing the gene encoding for TGF-β1 into three adjacent lumbar discs of healthy rabbits. The superior and inferior adjacent discs were used as controls. The injected discs were successfully transduced with the gene through the large increase in TGF-β1 production as compared with the controls (Fig. 20.3). The experimental discs also showed an increase in newly synthesized proteoglycans by 200%. Thus, this study showed not only the feasibility of transduction of NP cells, but also the subsequent ability of those cells to produce increased levels of extracellular matrix. Lim mineralization protein-1 (LMP-1) is yet another candidate gene for gene therapy. LMP-1 is a growth factor that stimulates the production of both BMP-2 and BMP-7, both of which have previously shown promise in biologic therapy of the IVD.2,3,6,12 Yoon et al14 evaluated this both in vitro as well as in vivo using healthy rabbits. In vitro LMP-1 was found to significantly increase levels of both BMP-2 and BMP-7 expression, as expected. The hypothesized downstream effect of increased proteoglycan production was seen as well. Additionally, the use of a natural BMP inhibitor, noggin, was shown to effectively block these effects, a point of importance when considering the safety of gene therapy. Finally, rabbit IVD cells were successfully transduced with the LMP-1 gene using an adenoviral vector. Again significant increases in BMP-2, BMP-7, and proteoglycan, specifically aggrecan, were seen as compared with control discs. Although neither the effects of LMP-1 nor TGF-β1 have been tested in vivo using a disc degenerative model, the results suggest that both growth factors could be an additional useful growth factor for gene therapy of DDD.
Injection Therapy
Bone Morphogenic Protein-7 (OP-1)
Growth and Differentiation Factor-5
Summary
Gene Therapy
Vectors
In vivo Gene Therapy
Bone Morphogenic Protein-2
Transforming Growth Factor β-1
Lim Mineralization Protein-1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








