Fig. 23.1
Comparative graphic of the amphetamine and phenethylamine molecules. Note the similarity of the molecular structure
Other groups have reported a reduction in phenylacetic acid levels measured in urine, plasma, and cerebrospinal fluid (CSF). It has been proposed as a state marker. In the same way, changes in the levels of these compounds in addition to antidepressant treatment have also been suggested as state markers for depression. It has been proposed that an impaired p-tyramine conjugation after a tyramine challenge could be considered an acceptable depression trait marker [30].
Neuroendocrine Windows
Beginning from the idea that mental illness in a strict sense is due to nervous pathways disorders and the fact that it can be studied in the same form that neuroendocrine pathologies are, a neuroendocrine windows model was generated. It was proposed as a way of accessing what happens within the “black box”, sending a stimulus and expecting a measurable response. Neuroendocrine research strategies in biological psychiatry began in the 1960s, but they shone in the 1980s.
These neuroendocrine studies started from various experimental observations and from diverse clinical and basic facts [2, 5, 31–33]. There is an overlap or co-activation of the formations involved in the regulation of emotions and hormonal secretion patterns at the level of brain structures. Relations between the limbic system and stress activation patterns are known. These reactions are mediated by the same neurotransmitters, and the relationship between the conventional neurotransmitters and hypothalamic releasing factors have been extensively studied by other teams as well as our group (see Fig. 23.2).
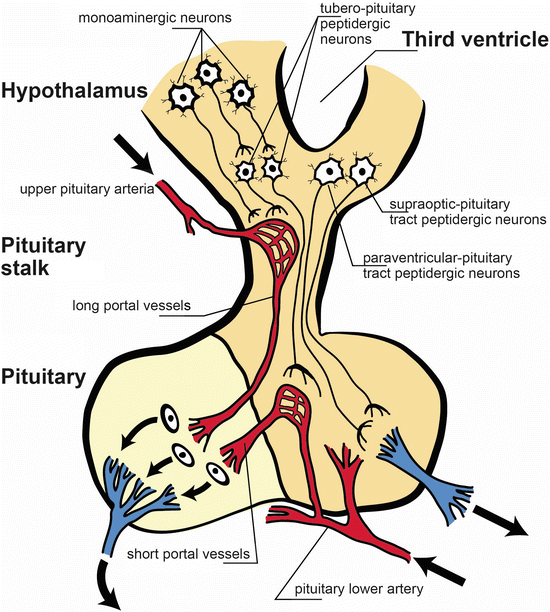

Fig. 23.2
Anatomical and functional scheme of the hypothalamic-pituitary axis. The pituitary stalk stem connects the hypothalamus to the pituitary. Monoaminergic neurons activate releasing factors peptidergic neurons (tubero-pituitary peptidergic neurons), and they exert its action on the adenohypophysis. Peptidergic neurons from supraoptic-pituitary and paraventricular-pituitary tracts project to neuro-pituitary. Note the pituitary lower artery and the vessels emerging form it, the short portal vessels. The long portal vessels are released form the upper pituitary artery [Modified from the Ph.D. Thesis of Prof. Dr. Pascual Angel Gargiulo [1],. and Gargiulo, P.A.: “Psiconeuroendocrinología”. En: Vidal, G.; Alarcón, R.D.; Lolas Stepke, F. (Eds.): Enciclopedia Iberoamericana de Psiquiatría. Vol. III: 1376-1386. Editorial Médica Panamericana. ISBN: 950-06-5054-1. Buenos Aires. 1995 [2]. (With permission from Editorial Médica Panamericana)]
In many cases the hypothalamic releasing factors also have endocrine and behavioral functions and eventually, psychotropic effects [31–33]. In turn, hormones can have obvious psychotropic actions ([34, 35], see Figs. 23.3, 23.4, 23.5, and 23.6). On the other hand, psychotropic drugs can alter hormone levels [38–40].
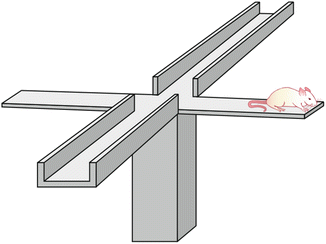
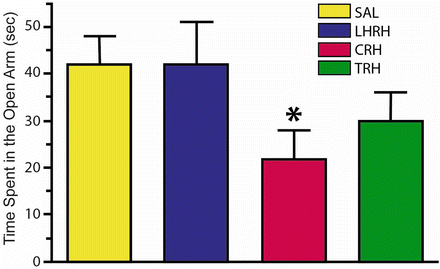
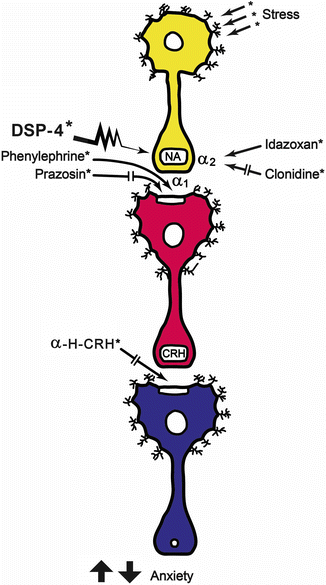
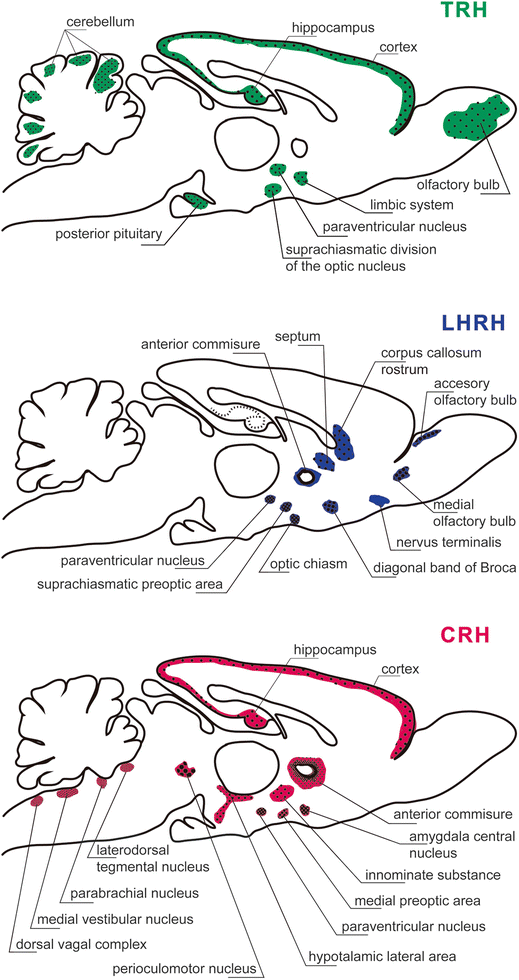

Fig. 23.3
Schematic representation of the plus maze test. The rat is placed on the extreme of the open arm extreme [Modified from the Ph.D. Thesis of Prof. Dr. Pascual Angel Gargiulo. [1], and Gargiulo, P.A.: “Psiconeuroendocrinología”. En: Vidal, G.; Alarcón, R.D.; Lolas Stepke, F. (Eds.): Enciclopedia Iberoamericana de Psiquiatría. Vol. III: 1376-1386. Editorial Médica Panamericana. ISBN: 950-06-5054-1. Buenos Aires. 1995 [2]. (With permission from Editorial Médica Panamericana)]

Fig. 23.4
Time spent in the open arm in the plus maze test injecting saline, luteinizing hormone releasing hormone (LHRH), corticotrophin releasing hormone (CRH) and thyrotrophic-releasing hormone (TRH) via the intracerebroventricular (ICV) method. Note the significant decrease in time spent in the open arm induced by CRH [Modified from the Phd Thesis of Prof. Dr. Pascual Angel Gargiulo [1], and from Gargiulo and Donoso [36]. (With permission from The Brazilian Journal of Medical and Biological Research)]

Fig. 23.5
Schematic representation of the findings of Berridge and Dunn [37]. A decrease in exploratory behavior can be seen when stress activates noradrenergic neurons, which could be considered as an anxiety index. The α-2 auto-receptor blockade using idazoxan potentiates the stress response. The stress response was decreased by clonidine (α-2 agonist) and DSP-4 (a noradrenergic selective neurotoxin). It can be concluded that facilitation of noradrenergic response increases stress responses, and the antagonisms decrease them. Postsinaptically, and taking into account the postsynaptic neuron, stimulation of the α-1 receptor of the CRH neuron, the stress response can be elicited. The prazosin blockade of this receptor interfere the stress response. The postsynaptic blockade of CRH actions by the antagonist α–helical-CRH decreases the parameters related to stress response [Modified from the Ph.D. Thesis of Prof. Dr. Pascual Angel Gargiulo [1],. and Gargiulo, P.A.: “Psiconeuroendocrinología”. En: Vidal, G.; Alarcón, R.D.; Lolas Stepke, F. (Eds.): Enciclopedia Iberoamericana de Psiquiatría. Vol. III: 1376-1386. Editorial Médica Panamericana. ISBN: 950-06-5054-1. Buenos Aires. 1995 [2]. (With permission from Editorial Médica Panamericana)]

Fig. 23.6
Extrahypothalmic localization of the peptidergic-releasing hormones thyrotrophic releasing hormone (TRH), luteinizing hormone-releasing hormone (LHRH), corticotrophin-releasing hormone (CRH) in hypothalamic and extra-hypothalamic regions. It should be appreciated that TRH neurons are present in the olfactory bulb, cerebral cortex, hippocampus, limbic system, suprachiasmatic division of the optic nucleus, paraventricular Fig. 23.6 (continued) nucleus of the hypothalamus, posterior pituitary, and cerebellum. There are LHRH neurons distributed in accessory olfactory bulb, medial olfactory tract, nervus terminalis, diagonal band of Broca, rostrum of the corpus callosum, septum, anterior commissure, optic chiasm, hypothalamic paraventricular, and suprachiasmatic preoptic areas. The CRH neurons are present in the cerebral cortex, hippocampus, anterior commissure, innominate substance, amygdala central nucleus, medial preoptic area, hypothalamic paraventricular nucleus, lateral hypothalamic area, perioculomotor nucleus, laterodorsal tegmental nucleus, parabrachial nucleus, medial vestibular nucleus, and dorsal vagal complex [Modified from the Ph.D. Thesis of Prof. Dr. Pascual Angel Gargiulo [1],. and Gargiulo, P.A.: “Psiconeuroendocrinología”. En: Vidal, G.; Alarcón, R.D.; Lolas Stepke, F. (Eds.): Enciclopedia Iberoamericana de Psiquiatría. Vol. III: 1376-1386. Editorial Médica Panamericana. ISBN: 950-06-5054-1. Buenos Aires. 1995 [2]. (With permission from Editorial Médica Panamericana)]
Endocrine illnesses induce psychiatric disorders. Thus, hypothyroidism can generate a symptomatic depression [41, 42] and, in turn, patients with major depression have a higher risk of hypo- or hyperthyroidism [43]. Finally, psychiatric disorders such as depression, characterized by high levels of cortisol [44], may trigger endocrine disorders such as Cushing disease [45, 46]. In some cases, neuroendocrine tests have been associated with immunological markers, aiming to increase sensitivity using them as state markers [47].
The scope of the relationship between the neurotransmitters and hormone release was one of the biggest contributions of our country. It decisively contributed to correlate the behavior to endocrinology and neurotransmission [48]. From these premises, we postulated some examples about the possibility of scrutinizing the efferent of central circuits involving hormones, using the concept of “windows” [1, 2]. Thus began the study of mental disorders through neuroendocrine tests. After numerous initial attempts, some of them reached the stage of clinical use. They were considered “windows” to study some relevant circuitries of the brain, which were the dexamethasone suppression test (DST), thyrotropin releasing hormone (TRH)/ thyroid stimulating hormone (TSH) test, luteinizing hormone releasing hormone (LHRH) and luteinizing hormone (LH) test, and the clonidine test [44, 49–52]. A brief commentary is made at the end about cortisolemia and schizophrenia [8].
Dexamethasone Suppression Test
Among the biological changes in depressive disorder, an overactivity of the hypothalamo-pituitary-adrenocortical (HPA) axis has been signaled. The DST, as a reflection of HPA axis activity, has been the most thoroughly investigated “biological test” in psychiatry to date [53], and it has been proposed as a state marker for endogenous depression. There are some variables involved in the present interpretation of DST results and its relation to clinical symptoms [54].
The DST was first used in the 1980s beginning with the idea that corticoadrenal hyperfunction is the hormonal profile that better characterizes the depressed patients. It consists of an increase of cortisol in plasma and CSF, alterations in the circadian cycle, and increased urinary excretion of its metabolites [44]. To sensitize the diagnosis the DST was used, it was initially proposed to diagnose Cushing illness [55, 56], but the methodology was modified to adapt to psychiatric illness. Carroll applied a variation of the test to psychiatric patients, finding that a significant percentage of depressed patients had an early cortisol escape to dexametsone [44, 49].
The standardized form of the test consists of an oral intake of dexamethasone (1 mg) at 23 h, and performing two measurements of plasma cortisol at 16 and 23 h the next day [44, 49]. A cortisol level higher than 5 g/dl in one of the samples in this scheme was considered abnormal. According to some studies, it has been proposed in various publications that there should be a degree of specificity of 96 % and sensitivity between 40 and 60 %. Later studies have demonstrated a significant sensitivity in other psychiatric patients, with significant stress factors such as hospitalization, age, and weight loss [57].
Beyond the agreements and disagreements in the interpretation of clinical significance, the positivity of this test suggests a biological treatment, and it is an indicator of poor response to psychotherapy, and lack of response to placebos [58]. In turn, the normalization of the test can be observed before or after clinical improvement, and the continuation of a positive result is considered a factor of poor prognosis linked to suicide risk [59].
It was observed during a clinical trial that transient suppression of HPA function using dexamethasone suppression appears to reduce the exaggerated fear prevalent in patients with post-traumatic stress disorder (PTSD), showing a significant treatment effect in those subjects that was not observed in the control group [60]. It may be related to a negative feed back decreasing corticotrophin releasing hormone (CRH) activity, an anxiogenic factor [36] that can be overactive in this population.
An alternative test has been proposed using a 5 mg dose of prednisolone. The dose generates a partial HPA suppression, proposing assessment of salivary cortisol as a tool to use it in wide samples of psychiatric patients [61]. Prednisolone has additional effects on mineralocorticoid that seems to induce different effects in the same depressed patients and to give different biological and clinical information when compared with dexamethasone in depressed patients groups [62]. It has been proposed that severe treatment resistance in depressed patients is associated with a dysfunctional feedback response when glucocorticoid and mineralocorticoid receptors are simultaneously activated by prednisolone [63].
Another procedure that was derived from several recent studies, is the combined dexamethasone (DEX)/CRH test [64]. In its classic technique or method of implementing the combined dexamethasone (DEX)/CRH test [65] consists of an oral pretreatment with dexamethasone (1.5 mg, 11:00 p.m.), and on the next day, a CRH intravenous bolus (100 μg, 03:00 p.m.). A cortisol response curve is obtained, and it is possible to recognize mood disorder vulnerability and response to stressors [64]. It has been proposed as a marker in depressive disorders, but it has not been adequately studied, and new studies are necessary for this purpose [66]. This test has been proposed as a marker of antidepressant effects [67].
Using this test in normal people, the function of the HPA axis has been studied in conditions of psychological distress and coping styles [68] as well as during sleep. In this case poor sleep is accompanied by increased cortisol response [69]. In other study using the temperament and character inventory (TCI), and evaluating the HPA axis reactivity using the DEX/CRH test, it was observed that some psychological features diverged between incomplete suppressors and enhanced suppressors. This late group showed lower cooperativeness and higher reward dependence as significant predictors of an enhanced suppression [70]. These findings suggest that personality subtypes condition different patterns of cortisol reactivity, turning relative and downplaying a purely biologically interpretation of this test, leading to a detailed consideration of the importance of individual psychological factors in biological findings [70].
TRH/TSH Test
Similar to the HPA axis, the hypothalamo-pituitary-thyroid (HPT) axis has been also investigated in depression. The TRH is a hypothalamic tripeptide, which in humans stimulates the release of prolactin and TSH, and in some cases growth hormone [71]. It was observed that depressed patients had decreased or absent TSH response to TRH [72], making it a new “window” for the study of psychiatric conditions. In its most widespread form, it is performed in the morning, fasting, by the intravenous injection of 200 to 500 mg of TRH. A blood draw is done prior to the injection (T0), and then blood is taken again at 15 and 30 min. On another schedule, periodic blood draws are done completing a 3 h curve. The expected response is a hormonal TSH peak at 30 min. It is considered a pathological response when this peak has a maximum below TSH 5 mUI/ml [50] or 7 mUI/ml [73]. A sensitivity of 40–50 % has been postulated according to the study and the diagnostic criteria used in them [74, 75]. After various studies, this test doesn’t allow differentiation between uni- or bipolar depression [51, 76]. The general status, the hormonal status, stress factors, addictions, and psychiatric drugs may have a significant impact on the results.
Most studies have focused on the TSH response to exogenously administered TRH. In those studies, blunted TSH responses have been found in depressives compared with normal controls. However, the frequency of blunted responses in other types of psychiatric patients has made this test marginally useful for differential diagnosis [53]. Additionally, in some cases an exaggerated response of TSH to TRH stimulation in a group of depressed patients with normal basal TSH level has been reported. The value of this test is relative, and limits its validity to some subgroups that must be determined [77]. When a comparison was made between normal controls, agoraphobics, and depressive and panic patients, a significantly higher response was observed in TSH response to TRH in the first two groups, suggesting a lower biological component regarding HPT axis in agoraphobia [78]. Responses to TRH in females appear to be higher when compared with males in both TSH and prolactin (PRL) levels [79].
There is no consensus on whether this test is a marker of status or rank, and it appears that no relationship exists between the abnormality and treatment response [51], although some studies suggest that a persistent alteration appears to be a predictor of relapse and suicide risk [80, 81]. Its use in suicide risk has been a matter of numerous studies with different conclusions.
Early studies suggested that neurochemical measures and neuroendocrine tests, independent of clinical diagnoses, may be used with the goal for exploring human aggression and suicide, including the TRH/TSH test [82]. Later, in a larger patient population, studying the possible predictive value of a reduced TSH response to TRH in patients followed along 6 years, it was observed that a smaller group that felt in suicide. They had a lower response than those that did not commit suicide. However, the difference did not reach statistical values, suggesting that this test cannot be used as a predictive parameter for suicide [83]. The hypothesis of a reduced TSH response to TRH was studied in a smaller group of euthyroid primary unipolar depressed female patients. They were tested using the TRH/TSH test while receiving psychiatric treatment. The study showed that the simultaneous presence of a symptomatic constellation integrated by panic, agitation, and suicidality in a depressive state may be correlated with the greatest reduction in TSH response [84].
Recently, the search on the use of this test in suicide risk was continued. In a study performed in a reduced group of male suicide attempters compared with healthy volunteers, no correlation was observed between TSH response to TRH and violent suicidality or a subsequent suicide. However, cerebrospinal homovanillic acid levels were related to TSH response in the suicide attempters group, suggesting a role for a failure of dopaminergic regulatory mechanisms in suicide attempters. Negative correlations were observed between T3 levels and suicide and depression scales used [85]. An association was established between history of suicide and the degrees of HPT axis dysregulation in a large sample of depressed patients [86]. Controversial findings give substance to this topic.
Using a combined registration system, and considering nocturnal hormones secretion and electroencephalogram (EEG), it has been observed that thyrotropin was decreased, and adrenocorticotropin hormone (ACTH) was elevated at initial time of sleep compared with controls, suggesting a negative correlation between HPT and HPA axes. These findings allow using these parameters as a ratio between TSH/ACTH in the first half of the night [87]. This related disturbance has been attributed to an impaired effect of TRH-related corticotropin-release-inhibiting-factor, trying to explain the negative correlation between both systems [87]. However, other groups, using other schedules, did not observe a clear interrelation of abnormalities between the axes (HPT and HPA) in depression [79], and some clinical experimental evidence strongly suggests that different biological dysfunctions could be underlying different markers, such as sleep EEG or hormonal disturbances in depressive patients [88, 89]. Technical differences may explain this contradiction and the phenomenon could be present only in the early state of sleep. Some clinical experimental evidence strongly suggests that different biological dysfunctions could be underlying different markers, such as sleep EEG or hormonal disturbances in depressive patients [88, 89].
This test has been used to evaluate treatment responses. The therapeutic action of electroconvulsive therapy (ECT) in depression appears not to be directly related to its effects on the HPT axis because ECT in depressive patients did not modify the response of the TRH/TSH test. Independent of the acute effect, a delayed effect of ECT on the HPT axis function cannot be excluded [90]. Finally, studies have been done regarding potentiation of antidepressants, and recently, selective serotonin reuptake inhibitors (SSRI) have been included in these clinical trials. In this way, concomitant administration of T3 to nonresponders, SSRI treated depressed patients improved the mood scores, starting from normal values in TSH and TRH/TSH test before T3 addition [91].
The endocrine tests were used also in schizophrenia, where higher basal TSH and GH levels appear to be a predictor of a poor treatment response [92]. However, some parameters could be considered markers of a good response, such as higher T3 levels, blunted TSH response to TRH stimulation, and positive DST [92].
LHRH/LH Test
It has been postulated that major depressive disorder is associated with abnormal regulation of LH secretion, and it has been argued that this LHRH abnormality can explain the fertility decline in depressive patients [50]. LHRH antagonists induce major depressive and panic attacks in women without depression [93]. However, other studies suggest a preserved normality on the hypothalamic pituitary gonadal (HPG) axis in endogenous depressed patients [94] and in depressed patients in general [95]. This evidence generates reasonable doubts about the exploration of this axis in depressed patients.
Clonidine/Growth Hormone Test
The clonidine test explores the release of growth hormone (GH). GH release is stimulated by dopamine, noradrenaline (α-2 receptors), and possibly serotonin. It has been theorized that noradrenergic inhibition is mediated by β receptors and gamma amino butiric acid (GABA) [96]. Clonidine stimulates GH secretion in normal subjects through α-2 receptors [97]. This test evaluates the response of GH to clonidine, a central agonist of α-2 adrenoceptor agonist. The most widespread technique involves the intravenous injection of 150 mg in 10 min, and GH is measured over 120 min. In normal people a GH peak between 30 and 60 min is expected. A pathological response to GH is considered when this procedure originates a peak below 5 ng/ml. The criterion also includes baseline rates below this level [52, 98]. A decrease of GH response to clonidine in patients with major depression has been reported compared with healthy subjects or minor depression [99–102]. To our knowledge, there are no studies of large groups. Its sensitivity is questioned [103–105]. Nonspecific factors also appear to play into their results [106, 107].
The proposed underlying disorder is reduced by postsynaptic α-2-adrenoceptor sensitivity and responsiveness [100, 108], and it may be accompanied by an increased presynaptic noradrenergic availability [100]. Blunted GH responses to clonidine observed in depressive patients are not attributable to pituitary GH secretion defects [109]. A consensus can be considered in the sense that noradrenergic and dopaminergic neurotransmitter disturbances are present in major depression, and an individual variability appears to be present regarding biochemical anomalies [102]. A study of a large group of patients showed that patients with affective disorders such as major depressive disorder or schizoaffective disorder, showed a blunted response when compared with controls or schizophrenic patients [110].
A decreased response in patients with major depression has been reported[52]. Some findings also support the hypothesis of decreased noradrenergic receptor sensitivity in unmedicated male patients with a nonendogenous major depressive episode [111]. A reduced GH response has been reported in depressive patients when compared with neurotic depressives, schizophrenics, and controls. It has been interpreted as a trait marker or vulnerability factor in endogenous depression, and it would be the case for unipolar or bipolar illnesses.
It has also been proposed as a tool to evaluate differential diagnostics in psychiatry. In this way, it has been reported a study in which normality of HPA axis responses to the Trier Social Stress and GH responses to clonidine were found exclusively in depressed patients. Patients with only anxiety showed a profile with normal HPA responses but the GH response was blunted. An elevated HPA and blunted GH response were observed when comorbidity was present [112]. Concurrent use of biological markers such as rapid eye movements (REM) sleep latency and endocrine tests may improve accuracy of diagnosis [113].
Endocrine status appears to have some influence. Hypercortisolemia, evaluated by DST, appears to not inhibit GH response to apomorphine or clonidine, suggesting that HPA axis in overactive depressed patients does not explain GH abnormal responses in major depressed patients [114]. Additionally, a lower GH response was observed in DST nonsuppressors, suggesting a correlation between positive DST and α-2-adrenoreceptor dysfunction [115]. Evidence suggests that gender and menopausal status are very important in GH test interpretation. In male patients diagnostic groups differed in the GH response to clonidine, but in female patients an additional difference was observed between the premenopausal and postmenopausal state, as was evident in different diagnostic groups [116].
This test is considered as a trait marker of depression because the alteration is maintained after healing [117, 118]. A pathological response does not predict a better outcome by treatment with adrenergic or serotonergic antidepressants, whereas a normal response has been regarded as a good argument for choosing a serotonergic antidepressant [119].
Therapeutics do not modify results. Postsynaptic α-2-adrenergic responsiveness is not enhanced after chronic antidepressant treatment because GH response is not modified [120]. It has been postulated that the effect of antidepressant treatments is mediated by a decrease of sensitivity of the α-2 adrenergic autoreceptor. It was observed in a clinical study using amytriptiline that some related parameters such as plasma levels of norepinephrine metabolite 3-methoxy-4-hydroxyphenethyleneglycol, standing systolic blood pressure, and sedation were induced by treatment. These findings may indicate a subsensitization of α-2 adrenergic autoreceptors. However, no effects were observed in the GH response to clonidine [121].
Cortisolemia in Schizophrenia
While searching for biological markers useable as predictors of therapy efficacy in schizophrenia, it was reported that cortisolemia or its changes using dexamethasone and structural computed tomography parameters may have a high value in this sense. Furthermore, these parameters can be used as tools to measure variables related to vulnerability-stress model of schizophrenia [8].
Central Chemical Studies
In the 1990s, the investigations were focused on the central study of the behavioral effects of pharmacological manipulation of delimited nuclei through experimental stereotactic procedures and its correlation with the findings of brain images. In this way new advances were made using translational and electrophysiological studies, starting in the clinic. The clinical counterpart has made an important number of new techniques that allowed the receptor concentration in brain nuclei and its modification by treatment.
Translational Approaches
Our team, which had projected to behavioral and neuroendocrine research using stereotactic techniques (see above), focused in the decade of the 1990s and the following years on the study of the neural basis of perception, cognition, and anxiety with a translational criteria based mainly in stereotactic access to various brain nuclei. This methodology had significantly lower costs when compared with the imaging studies. It allowed accessing and manipulating neurotransmitter systems in discrete brain areas, assessing its impact on the behavioral and neuroendocrine level. In some cases translational studies were designed beginning with previous clinical studies detecting the area involved in psychiatric disorders, to then generate a model of translational approach, which in turn brought additional pharmacological details to the imaging study. Therefore, we worked in translational models of anxiety, depression, and schizophrenia.
In our translational approaches to anxiety, our lines gave relevant data about various neurotransmitter systems involved in anxiety in different brain areas, including amygdala [33, 122, 123]. Nucleus acumbens septi (NAS) [124, 125] and the NAS-projecting structures such as the amygdala, medial prefrontal cortex, and hippocampus [123] established potential interactions between these structures in modulating anxiety. In that paper we compared our findings with data from neuroimaging in anxiety disorders [123].
We have experimentally addressed both the schizophrenic and the affective psychoses in the psychoses. In our approaches to depression we mainly studied the role of tracer amine precursors as antidepressants, as referred to above [27], with different lines and ongoing clinical translational approaches. In the area of schizophrenic psychoses, we started our clinical studies of perceptual disturbances in schizophrenic patients [126, 127]. This led us to propose schizophrenic psychoses translational models such as models inducing primary symptoms [128, 129], secondary or negative symptoms [125], and cognitive symptoms of schizophrenia [124, 130, 131] acting prevalently on glutamatergic transmission of NAS.
EEG Mapping and Event-Related Potentials
Cerebral biophysics made its most significant and stimulating contributions in the decade of the 1990s from a clinical standpoint [132, 133]. The introduction of evoked potentials (EP) and quantified EEG mapping began a continuing road with emission tomography. The start was linked predominantly to biophysics, but studies of receptors led to a remarkable biochemical refinement. After 35 years of images in psychiatry, it has achieved a remarkable level of spatial resolution, both temporal and molecular [134].
Recent advances in EEG processing and analysis may provide high resolution functional brain imaging with interesting spatial and temporal detailed information [135–137]. These techniques may incorporate relevant data for the study of psychiatric disorders [136]. Because of several clear advantages such as simple use, low cost, and noninvasive acquisition characteristics, it became an interesting research and clinical psychiatric tool, increasingly used as neuroimages, constituting a way to search for future diagnostic biomarkers and not merely an oscilloscope or a spike-counter [135–137]. It has been said that as magnetic encephalography (MEG) is an instrument designed for recording magnetic brain fields, EEG measures electric potential fields [135]. These EEG techniques have been concurrently used with functional magnetic resonance imaging (fMRI) and MEG aiming to obtain a multimodal functional neuroimaging [137]. Some strategies have been proposed with the goal of improving images in a probabilistic mode and delineating algorithms in neurological and psychiatric diseases such as Alzheimer and schizophrenia [138].
The quantified EEG (QEEG) mapping, a sophistication of the EEG, was one of the first techniques used largely in the 1990s in this newly initiated development, starting the imaging studies in psychiatry [139]. This new method was ahead of conventional polygraph allowing the anatomic correlation. Soon numerous studies appeared showing markers in anxiety disorders, affective disorders, schizophrenia, dementia, and other disease entities, and continues at present [140, 141]. Another important study emerged from that initial stage of EP linked to an event (event related potentials, ERP). It’s most well known is the detection of auditory stimuli [142]. Cognitive EP and mapping QEEG have enabled interesting and accessible approaches in professional practice [132].
Anxiety Disorders
In anxiety disorders, the QEEG has shown to have utility as a marker, and has shown instability in the levels of cortical “arousal”, perceptible in the register [143]. Also common to most anxiety disorders are specific difficulties in the sensory input conditions and the allocation and utilization of attention, which is evident in EP and ERP [144]. These alterations can be observed in all manifestations of anxiety and distress, such as obsessive-compulsive disorder, generalized anxiety disorder, panic attack, phobias, and PTSD as prevalent anxiety manifestations [143].
Affective Disorders
In affective disorders, the QEEG has been proposed as a selection criterion for selecting the antidepressant drug [144–146]. Comparing antidepressant drugs, it has been proposed that it is important to consider gender in the studies of drug treatments. It was proposed in the same study that the higher sigma frequency range of non-REM sleep and REM density, registered during the sleep EEG, could be used as markers of drug efficacy [147].
A combined approach using sleep EEG and hormonal secretion patterns has been tried, measuring testosterone and cortisol in depressive patients. The patients were diagnosed with major depressive disorder, and values were considered during the illness and after remission. A blunted testosterone level and an increase in cortisol was observed, suggesting an interaction between both axes involved in this interaction (hypothalamic-pituitary-gonadal and limbic-hypothalamic-pituitary-adrenocortical axis) [148]. The overactivity of the HPA axis is a well-known fact [53]. This phenomenon could be explained starting from previous basic experiments [149, 150]. CRH injected within medial preoptic area (mPOA) decreases plasma LH levels in rats, not modifying follicle-stimulating hormone levels [149]. The mPOA and the hypothalamic arcuate nucleus are involved in this regulation [148, 149]. Stressors may activate HPA axis and suppress the HPG axis, inducing an antireproductive effect [148, 149].
Some reports have focused on cognitive functions for both schizophrenia and bipolar disorder, aiming to identify phenotypes and even common markers [151, 152]. It has been reported that relatives of people with depressive disorder showed an increased activation of brain-related zones during a verbal working memory task, registered by functional MRI (lateral occipital cortex, superior temporal cortex, and superior parietal cortex) [153].
Schizophrenia
Anatomical Images
Anatomical images have been used as markers in schizophrenia. Hippocampal volume loss has been reported in early schizophrenia, is not shared by healthy siblings, and appears to be related to the course of the illness, as an important intermediate phenotype of the illness, and proposed as trait markers [154]. Using a mix of images and neurological checking, smaller left dorsolateral prefrontal cortex volume and some exaltation of primitive reflexes at baseline may be useful tools to predict enhanced negative symptoms, suggesting that neurological soft signs could be clinically used as a mean to evaluate prognosis of schizophrenic patients [155].
Cognitive Functions
From our earlier work on the subject the importance of perceptual distortions in schizophrenia has been increasingly valued [126]. Particular deficits in masking tasks in schizophrenic patients have been reported in experimental approaches. These deficits consist of short-term visual stimuli followed by masking stimuli. This latter stimulus interferes with the perception, and this difficulty is higher in schizophrenic patients. This problem has been attributed to deficits in cholinergic transmission center [156]. It is also correlated to auditory perception deficits of schizophrenic patients with deficits in the consistency of high and low bands of gamma activity, postulating that disconnection would relate to the processing of auditory stimuli [157]. Auditory hallucinations have been studied with various strategies [158], mainly by combining data from EEG and MEG. Research has shown a difficulty with interregional connections between the activity of the frontal and temporal lobe, among other phenomena [158]. Perception performance has also been used in schizophrenia as a functional marker. It has been suggested that certain visual deficient functions in schizophrenia, can be used in this manner. The goal is to establish trait or state markers for schizophrenia. It has been reported that motion integration is dysfunctional only in schizophrenics, and not in their relatives or bipolar patients. However, motion discrimination appears to be dysfunctional in schizophrenics and in their relatives, as well as being normal in bipolar patients [6]. These findings have relevant value because they allow the distinguishing of trait markers from transient state markers in schizophrenic patients using visual processes as markers [6]. Because abnormalities in visual scan paths have been reported in schizophrenic patients, scan path measures have been postulated as trait markers for schizophrenia [159].
Other groups have proposed relatively specific olfactory identification and spatial working memory deficits as markers that existed previously to illness onset. It has been suggested that they may be more potent as trait markers for psychosis than other cognitively tasks (i.e., verbal memory). A progressive declination could be expected with illness, the progressive steps of the illness, and have been proposed as states relative to trait factors [160].
With the goal of establishing stable trait markers for schizophrenia, an important number of studies have been displayed searching for neurocognitive deficits. It has been proposed that they may be detected before the initial manifestations of the illness, and could be present in the relatives of schizophrenic patients [161]. These premorbid cognitive deficits may be conditioned by brain abnormalities, giving the necessary basis for presentation of the illness, and the possibility of an early detection of the problem, mainly during adolescence, which is considered the age of maximal vulnerability for schizophrenia [161]. Studies pointing to other functions such as measures of attention regulation, working memory, episodic memory, and emotion processing have been proposed as tools to identify phenotypes with cognitive disturbances related to schizophrenia and bipolar disorder [151]. There has been reported evidence about attentional and executive impairments in patients with schizophrenia and in their unaffected first-degree relatives. It has also been theorized that the performance of bipolar patients does not significantly differ from that of schizophrenic patients. Using a neuropsychological battery, it has been observed that both groups and their corresponding relatives showed a marked deficit in time execution in the Stroop test when compared with healthy controls. These findings suggest the possibility of transnosographical markers for a shared familial vulnerability common to schizophrenia and bipolar disorders [152].
EEG Mapping and ERP
Interest in the biomarkers for abnormal parameters detection using QEEG is a current issue in schizophrenic psychoses, and the idea is that the modification of these biomarkers is a “target” for possible treatments [162]. In the same sense, and given the relationship between cannabinoids and psychosis, some approaches have tried to establish links and relationships. These psychoses-inducting effects exerted by cannabinoids, and the relationship between cannabinoids and schizophrenic psychoses, have inspired these studies. These drugs exacerbate the positive, negative, and cognitive symptoms of schizophrenia [163]. Following these ideas, some studies have explored the effects of acute and chronic use of cannabinoids using EP and related techniques [163].
Other studies, using numerous electrophysiological variables such as QEEG and ERP, have attempted to establish markers of positive and negative symptoms of schizophrenia, evaluated in scales and specific neuropsychological profiles [164]. This has led to suggest, even at the risk of oversimplifying the proposition, a relationship between negative symptoms and frontostriatal dysfunction or deficit. On the other hand, positive symptoms would be related to the dominant temporal lobe [164].
ERP are techniques that has also been used in psychiatry. First, it has been observed that aging leads to an increase in latency of P-300 [165]. In major depression, cognitive EP have been proposed as markers of suicide risk [166]. Attentional and cognitive problems in major depressive disorder appear to have a translation through a difficulty to discern significant stimuli from the environment. The mechanism by which this occurs has been associated with disorders of sensory processing (P200) and additionally with impaired context processing (N200/p300 complex). This could be an explanation for the attentional impairment observed in cases of more severe depression [167].
One of the most robust findings, reproducible and consistent in biological psychiatry, is the amplitude reduction of the P-300 wave in schizophrenic patients which allows it to sustain as the more trustworthy biomarker of disease [168, 169]. The fact that different brain generators can contribute to EEG findings has led to studies of correlation between hemodynamic changes studied by fMRI and electrophysiological studies [169].
While searching for trait markers in schizophrenia, it was observed that a P300 reduction is present in schizophrenics and non-psychotic siblings in different schedules [170]. Temporoparietal P300 amplitude reduction and frontal P300 amplitude increase seem to be quantitative phenotypes associated with increased risk of schizophrenia. Both measures can be useful for increasing the statistical power of genetic studies of schizophrenia [171]. Other approaches related to P300 wave have been used in schizophrenic patients. It has been postulated that when it is induced by a crying-face, it may be used as state marker. When it is induced by a smiling-face, it may be used as trait marker during recovery [172]. It has been studied in schizophrenic patients that auditory P300 is affected by both arousal and emotion, and a significant negative correlation was found between P300 amplitude and the values of negative symptom scores. It was concluded that P300 amplitude and area can be considered state markers with an aim to measure parameters modified by recovery in schizophrenic patients. It was concluded that the observed effects were mediated by attention and emotion levels [173]. Using a frontotemporal event-related EEG coherence as a measure of functional connectivity, an impaired interaction of the frontotemporal macro-circuit indirectly reflects genetically determined abnormalities of frontal and temporoparietal microcircuits, and these abnormalities in frontotemporal connectivity have been proposed as trait markers of genetic risk for schizophrenia [174].
The constancy of the P300 reduction in schizophrenia has served to give consistency to diagnose it and to sustain its biological condition, starting from the reality of the marker [168]. It has been stated that this finding is a state and trait marker, and appears to be sensitive to the course of the disease, interacting with persistent negative symptoms, decreased attention, and underlying brain changes [168]. In experimental studies of perception of own movements associated with visual event-related potential, it has been observed that schizophrenic patients have difficulty in recognizing their own movements, and that this is accompanied by changes in the ERP [175]. The risk of suffering schizophrenia has been also attempted to be measured using ERP [176, 177]. The electrophysiological patterns have been used to study acute and chronic effects of ketamine, linking in these schemes the first with acute psychoses and the second with cognitive deficits; they have been related to electrophysiological disorders in translational models [178]. Other approaches, carried also using EP, allowed to find multisensorial processing deficits in schizophrenic patients, suggesting an important role for this alteration in schizophrenia [179].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




