(a) NR2 antibodies (b, ×400) staining of 5 mm coronal slices from rat with MCAo (1 h of MCAo and 24 h of reperfusion): 1—infarcted area; and 2—contralateral area, NR2 mRNA expression in (b) cortex of MCAo rats, (c) hippocampus of MCAo rats; and (d) neuronal damage in induced cerebral ischemia
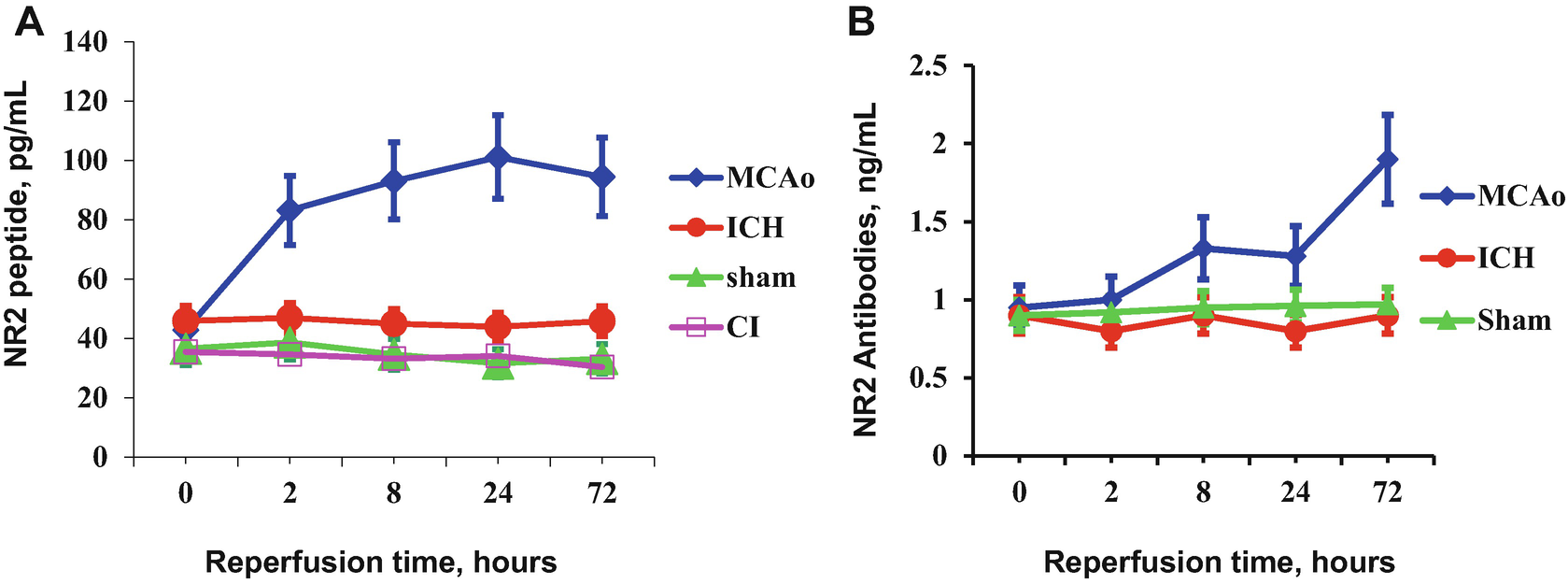
Accumulation of NR2 peptide (a) and NR2 antibodies (b) in the blood of experimental animals
The NR2 peptide release might be a tissue-based evidence of early neurotoxicity underlying transient cerebral ischemia. The circulating NR2 antibodies measured in the blood support the brain origin of NR2 peptide as a “foreign” antigen that is capable of activating the immune system to generate antibodies (Fig. 2b). Significant amount of NR2 antibodies appeared with a delay of 72 h in MCAo rats and persisted for weeks to months [6].
2.2 Glutamate Receptors in Induced Intracerebral Hemorrhage (ICH)
The role of NMDA receptors in ICH is unknown. Based on scattered reports of increases of glutamate in brain and CSF following ICH, Sharp et al. explored the possibility that local increases in blood flow might be related to glutamate release [10]. To examine the mechanisms of changes in glucose utilization, Sprague Dawley rats subjected to lysed blood injections into striatum were pretreated with NMDA antagonist MK-801. The results showed that [14C]-2-deoxyglucose uptake (injected intraperitoneally 1–72 h after ICH) reduced in the region of ICH, but increased in the perihematomal region, peaking at 3 h after the lysed blood injection [10]. The pretreatment with MK-801 blocked the increased glucose uptake produced by ICH. It was also demonstrated that NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) but not glutamate injections upregulated glucose uptake in perihematoma brain early after ICH and indicated the ionotropic glutamate receptor-dependent glucose hypermetabolism [11].
NR2 mRNA expression in the cortex of rats with intracortical rat blood cells (RBC) infusion was steadily decreased up to 47–56% at all time points studied in hematoma cortex compared with controls. Low concentrations of NR2 peptide in the blood samples of RBC-infused rats were accompanied by a reduced immune response (Fig. 2a). NR2 antibodies monitored in the blood sera of rats with intracerebral hematoma did not vary significantly compared to sham-operated rats (Fig. 2b).
The combined data suggest that NMDA receptor function is down-regulated after ICH compared to cerebral ischemia and the detection of NR2 peptide or antibodies in blood could be of benefit for discriminating between these two types of stroke.
3 NR2 Peptide Assays Development for Acute Stroke
3.1 NR2 Peptide Enzyme-Linked Immunoassay (ELISA)
The NR2 Peptide test is a magnetic particle-based enzyme-linked immunosorbent assay (MP-ELISA) intended for the quantitative determination of NR2 peptide fragment of NMDA receptors in plasma. The test is intended to be used in conjunction with clinical evaluation and radiological methods for diagnosis of acute ischemic stroke.
Concentrations of NR2 peptide are determined immunochemically in a blood assay.
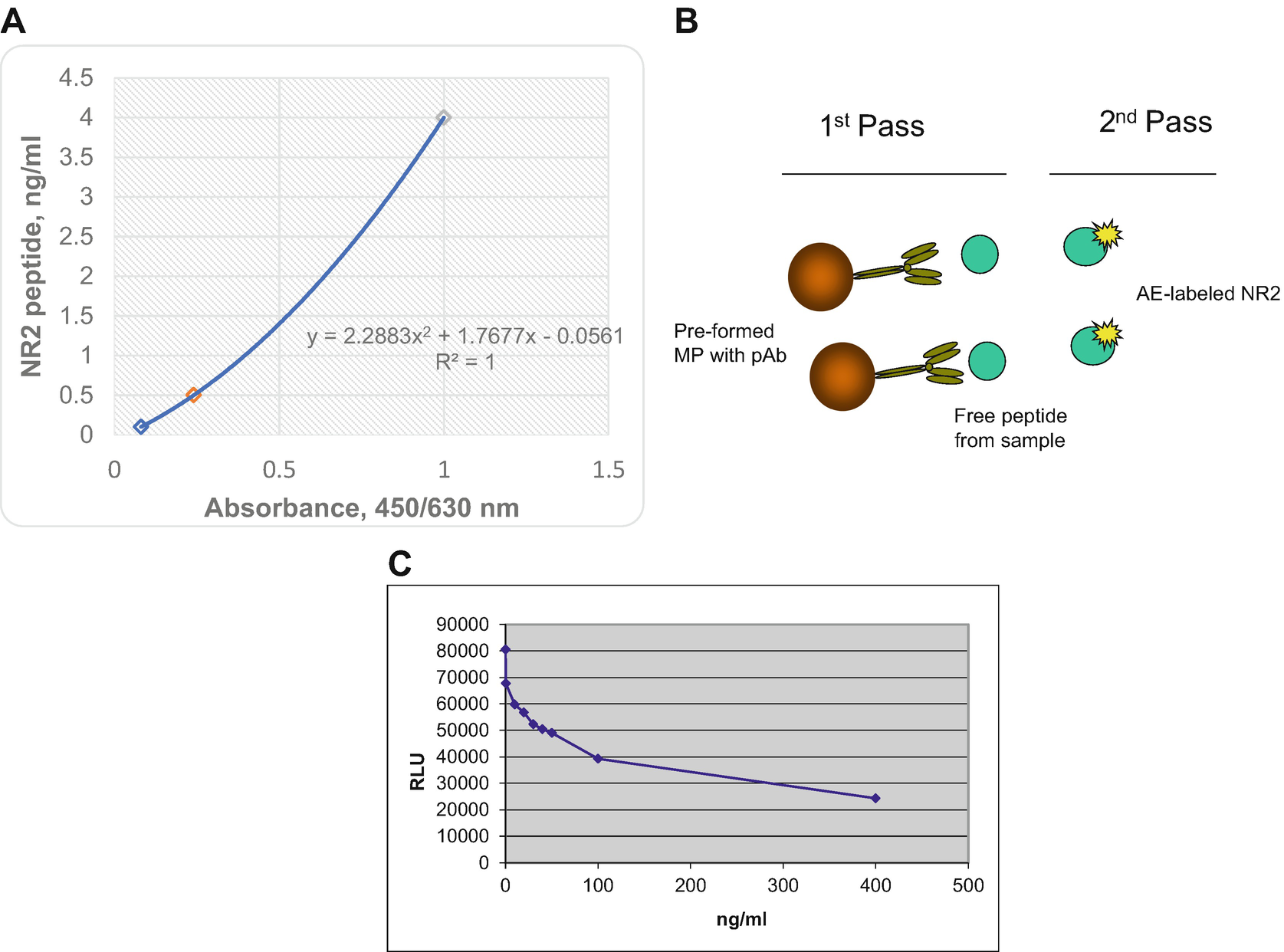
(a) MP-ELISA calibration curve of NR2 peptide assay, (b) scheme of automatic analyzer (AA) format for NR2 peptide detection, (c) NR2 peptide AA assay calibration curve where RLU—relative light units
3.2 NR2 Peptide Automatic Analyzer (AA) Assay
The assay transfer to automated analyzer platform employed paramagnetic particles and chemiluminescent technology. A modification is made to accommodate unique reagents for “on-board” detection using ready-to-use reagent packs. Preformed with streptavidin supermagnetic particles (0.8 μm) are bound to carrier antibodies (Ab) for the quantitative detection of NR2 peptide (Fig. 3b). The analyte in plasma competes with chemiluminescent labeled synthetic peptide for preformed with microparticles (MP)-polyclonal Ab (competitive assay). Translation from flat surface of microplate to microparticles increases reaction surface with simultaneous reduction in reagents consumption. That allows to speed up the turnaround time of laboratory testing from to 30 min.
Programmed AA separates, aspirates, and washes three times the cuvettes using buffer (PBS/Tween 20). Then it dispenses Base reagent to initiate the chemiluminescent reaction that automatically measured and reported in relative light units (RLU) according to software to the selected option, as described in the system operating instructions. Results of a standard calibration curve with RLU readings are shown on the y-axis against NR2 peptide concentrations (ng/mL) shown on the x-axis (Fig. 3c).
The anti-NR2 peptide assay kit uses a ready-to-use pack consisting solid phase compartment filled with 100 μg/mL latex paramagnetic MP preformed with biotinylated pAb. The second compartment of ready-to-use pack contains 10 ng/mL NR2—acridinium ester (AE) Lite reagent. The separate ancillary pack holds PBS-T buffer is necessary for reagent dilutions and pack for base reagents used to initiate the chemiluminescent reaction should be presented on-board of AA during the testing. Reagents in both packs are sufficient for 100 tests.
3.3 Nanoparticles Lateral Flow (nLF) Procedure for NR2 Peptide Detection
Recognizing the medical need of bedside testing for acute stroke, largely for neuro-critical care, point-of-care (POC) testing platform development has been initiated. Lateral flow based immunostrips engage gold nanoshells (150 nm) that dramatically increase sensitivity of detection reagents. The use of nanoshells results in a large visible signal even at low analyte concentrations.
The test principle is based on nanoparticles lateral flow technique and is a direct immunoassay [12]. The conjugate consists of nanoshells labeled with specific capture antibodies. NR2 peptide in the sample interacts with the conjugate to form a complex that migrates along the strip to the next section, which is the reaction matrix [12]. This reaction matrix is a nitrocellulose membrane, onto which the detection antibodies have been immobilized in bands to create test (T) and control (C) lines that serve to capture the NR2 peptide-conjugate complex and unbound conjugate, respectively. Excess reagents move past the capture lines and are entrapped in the wick or absorbent pad. Results are interpreted on the reaction matrix as the presence or absence of lines of captured conjugate, read either by eye or using a reader [12].
4 NR2 Antibody (Ab) Tests to Assess Chronic Ischemic Events
4.1 NR2 Ab ELISA Assay
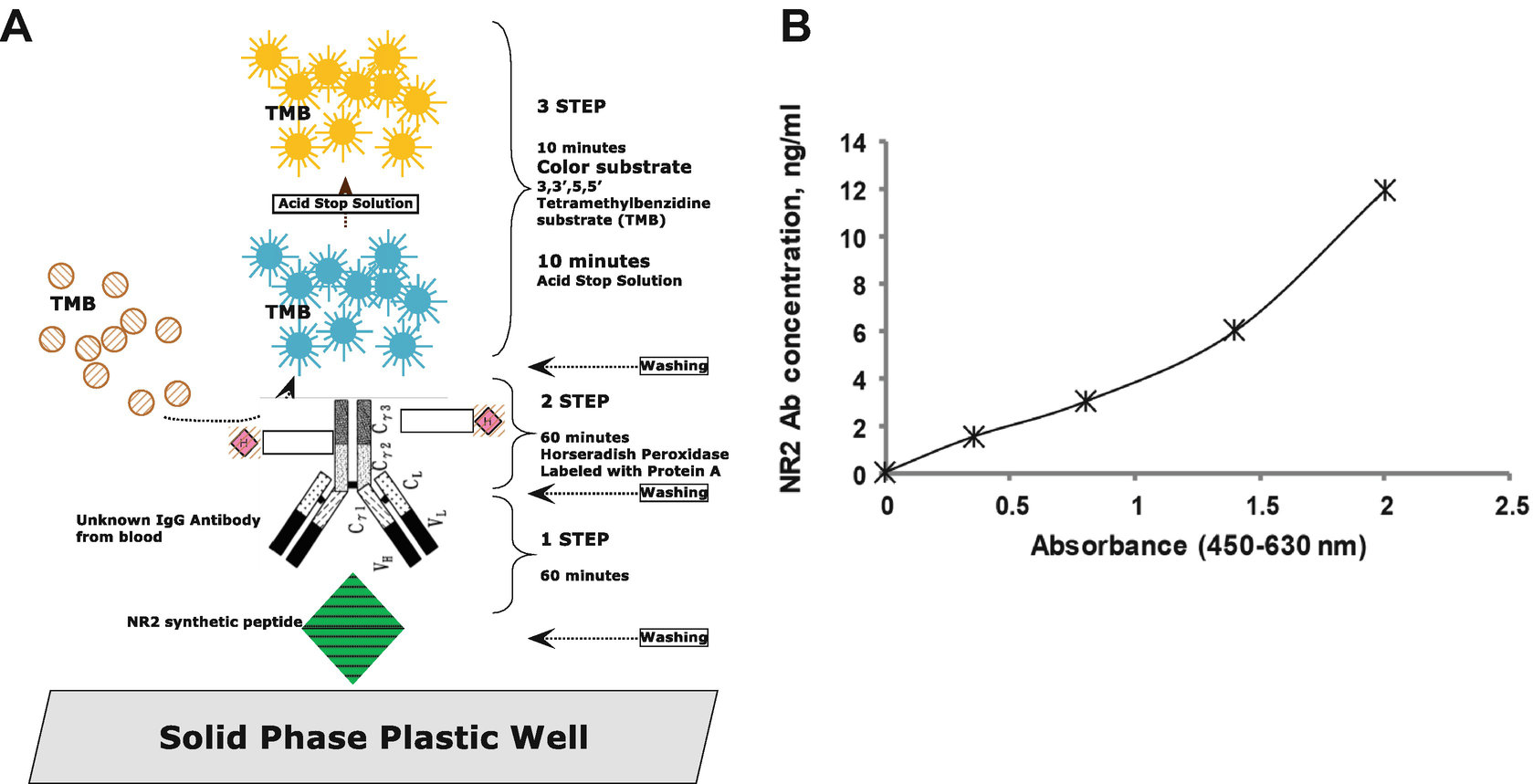
(a) NR2 Ab ELISA scheme, (b) NR2 Ab ELISA calibration curve
The detection of the formed immunocomplex—fixed on the MTP—is then determined by the HRP/tetramethylbenzidine (TMB)/H2O2-enzymatic reaction which is stopped by diluted acid. A color change from blue to yellow occurs after the addition of acid and the obtained color is measured photometrically at 450 nm referenced against 630 nm. Results of a typical standard calibration curve with optical density (OD) readings at 450 nm vs. 630 nm are shown on the x-axis against NR2 Ab concentrations (ng/mL) shown on the y-axis (Fig. 4b).
4.2 Automatic Analyzer Platform for NR2 Ab Assay
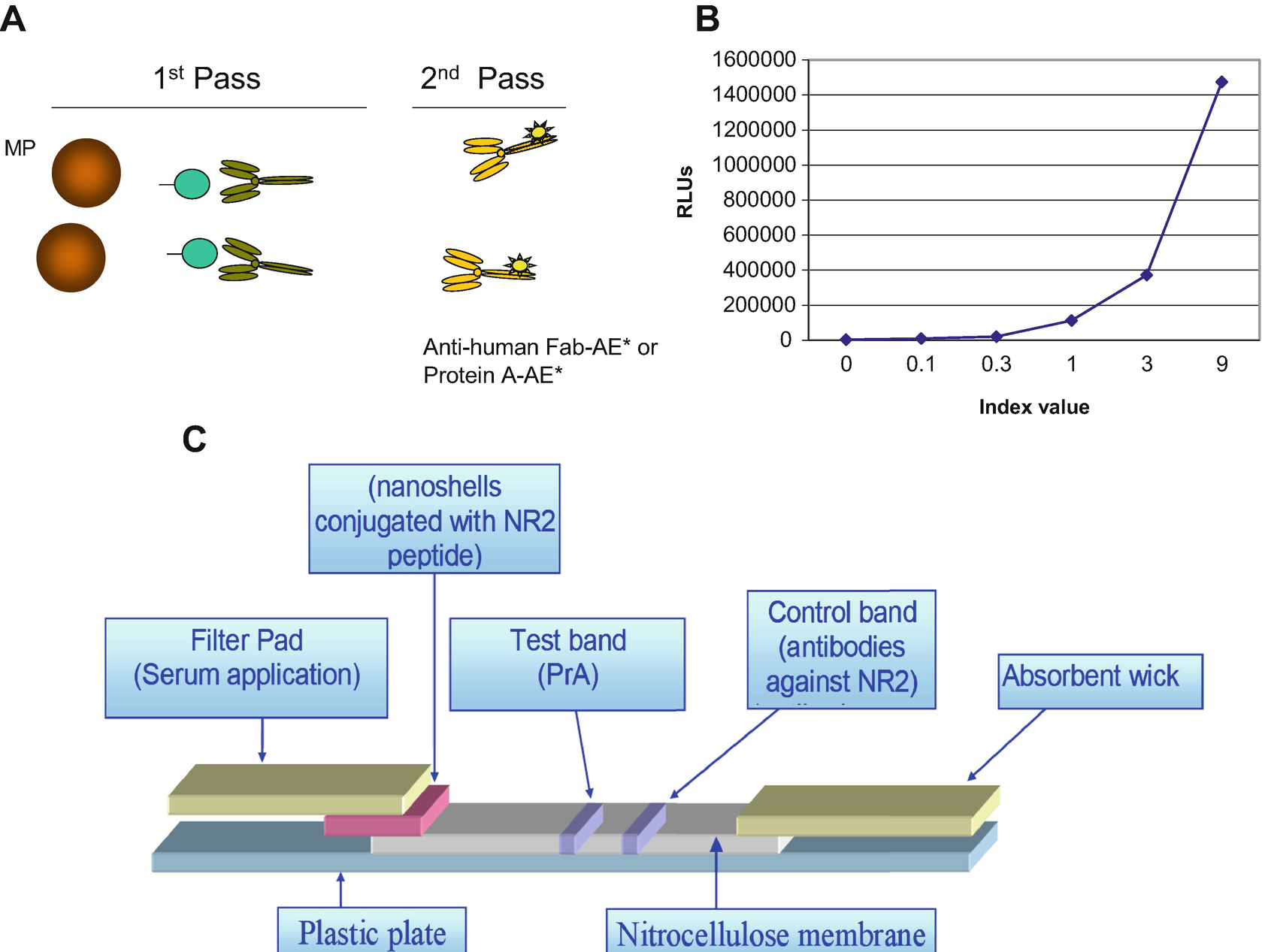
(a) NR2 Ab AA scheme, (b) NR2 Ab AA calibration curve, NR2 Ab nLF assay principle: strip configuration
The NR2 Ab assay kit uses a ready-to-use pack consisting solid phase compartment filled with 100 μg/mL latex paramagnetic MP. The second compartment of ready pack contains 0.5 μg/mL biotinylated NR2 peptide. The separate three ancillary packs hold (1) 10 ng/mL Protein A reagent, (2) buffer that is necessary for reagent dilutions, and (3) pack for base reagents used to initiate the chemiluminescent reaction. Reagents in all packs are sufficient for 100 on-board tests.
4.3 NR2 Ab LF Design
Figure 5c shows the configuration of nLF immunoassay for NR2 Ab detection in serum and whole blood. The assay consists of several overlapping zones, constituted by segments of various filters of different material and nitrocellulose mounted on a plastic card using an adhesive. To run a test, sample (20–30 μL) is added to the proximal end of strip, the filter pad where sample is treated making it compatible with the rest of test. Then sample migrates through this region to the conjugate pad containing gold nanospheres (80 nm) with attached modified NR2 peptide. The sample remobilizes the dried conjugate, and the NR2 Ab in the serum interacts with the conjugate as both migrate into the next section of the nitrocellulose strip or the reaction matrix. This reaction matrix contains one band of Protein A (test) and the second one of specific polyclonal or monoclonal antibodies against NR2 peptide (control band) where they serve to capture the analyte and the conjugate as they migrate by the capture lines. Excess reagents move past the capture lines and are entrapped in the wick or absorbent pad. Results are interpreted on the reaction matrix as the presence or absence of lines of captured conjugate, read either by eye or using a reader.
5 NR2 Peptide and Antibody Assays Analytical Data
5.1 Original NR2 Peptide and Antibody Stability in Blood Samples
The recovery study of neurotoxicity biomarkers in blood samples (plasma and serum) from individuals is performed at approximate storage times spent by: (1) phlebotomist as a daily routine of blood draws in in-patient wards with storage on cold pack (+4 °C, 4 h), (2) reference laboratory representative for regular samples pick up at medical office’s outdoor storage containers. There is an adjustment of time of storage (24 h) made at room temperature (RT) accounting summer temperatures on South East of USA (35–40 °C) and 12-h shift. (3) The point of storage at −20 °C for 2 months is chosen as an average time of temporary refrigerated storage for research purposes.
Summary of plasma stability
Parameters | +4 °C, 24 h | RT, 4 h | −20 °C, 2 months |
|---|---|---|---|
N | 38 | 41 | 15 |
Mean recovery, % | 85.5 | 78.6 | 90.4 |
Standard deviation (SD) | 7.4 | 8.1 | 6.1 |
Correlation coefficient (r) | 0.989 | 0.992 | 0.987 |
On the basis of these results for NR2 peptide biomarker, it is recommended specimen collection is made in tubes containing EDTA (not heparinized) with immediate storage on cold packs. Samples should be centrifuged (3500 × g, 4 °C for 5 min) within 1 h of blood drawing. For storage up to a week, processed samples are stored in an aliquot tube at or below −20 °C. For longer storage, freeze samples at –80 °C.
Processed plasma samples may be frozen and thawed only once without significantly affecting the amount of NR2 peptide. To transport samples, shipment boxes in bigger Styrofoam box filled with dry ice (−78.5 °C, pellets or slabs not less than 3 lb per box) should be prepared.
NR2 antibodies in samples are stable for up to 3 h at room temperature. Serum samples may be tested within 3 days of collection when stored at 2–8 °C. It is recommended collecting blood samples in serum collection tube (e.g., gel separation). Samples should be centrifuged and separated at 2500 × g for 5 min within 3 h of venipuncture. Store all serum samples refrigerated (2–8 °C) immediately upon collection; however, for storage up to 3 months, processed aliquoted serum samples should be frozen at or below −20 °C and for longer storage at –80 °C.
Processed serum samples may be frozen and thawed up to two times without significantly affecting the amount of NR2 antibodies. To transport samples domestically, samples could be shipped at 2–8 °C on ice pack. For international delivery, frozen samples should be shipped on dry ice.
5.2 NR2 Peptide/Antibodies Assays Cut Offs, Turnaround Time, and Stability
5.2.1 Assays Cut Off Values and Detection Limits
NR2 peptide assays. Cut off values for NR2 peptide test varies from 0.5 to 1.5 ng/mL depending on age (children or adults), acuteness of cerebrovascular event (hours since onset), and consequently from clinical setting (emergency room, acute care, surgery, physician’s office). The minimum detection limit is 0.1 ng/mL, as calculated by interpolation of the mean plus 2 standard deviations of 20 replicates of the 0 ng/mL NR2 peptide ELISA and AA assay as well.
NR2 antibody assays. The reference interval calculated from the samples (92%) was found to be 0.87–2.0 ng/mL according to guidance for establishing reference intervals NCCLS Standard C28-A2 (http://www.zxyjhjy.com/upload/attached/file/20170406/20170406120112_8797.pdf).
Recent studies [13, 14] have demonstrated an increased risk of cerebrovascular disease (associated with NR2 antibody values >2.0 ng/mL (upper 90% of populations with stroke-like symptoms) vs. <2.0 ng/mL (lower 90% of the studied populations with non-stroke and non-transient ischemic attack (TIA). Therefore, a more conservative approach for identifying individuals with a significantly increased risk for TIA and ischemic stroke attributable to NR2 antibodies may be the 90th percentile value of the population. The cut off for NR2 antibody test is 2.0 ng/mL.
The minimum detection limit is <0.5 ng/mL, as calculated by interpolation of the mean plus 2 standard deviations of 20 replicates of the 0 ng/mL NR2 Antibody.
5.2.2 Turnaround Time
Summary of turnaround times for various assay formats
Test | Sample | Format | Turnaround information | |
|---|---|---|---|---|
Number of tests | Duration | |||
NR2 peptide | Plasma | MP-ELISA | 89 | 1 h |
AA | 1200 | 1 h | ||
LF | 1 | <2 min | ||
NR2 Ab | Serum | ELISA | 89 | 2 h |
AA | 1200 | 1 h | ||
LF | 1 | <2 min | ||
5.2.3 Unique Reagents Stability
ELISA format. NR2 peptide unopened test kits should be stored at 2–8 °C up to 6 months upon receipt while NR2 Ab test kits may be stored refrigerated for 12 months. Opened test kits will remain stable until the indicated expiration date provided they are stored as indicated.
AA format. Both NR2 peptide and antibody reagent packs shelf life at 2–8 °C up to 6 months upon receipt. On “board” stability (inside working analyzer) for the same reagent packs is 8 h while NR2 antigen/antibody calibrator packs might be stored more than 14 days.
6 Bench Testing Studies
6.1 NR2 Peptide MP-ELISA and AA Performance Characteristics
Bench testing has been conducted to evaluate the performance of the NR2 peptide and antibody assays before use for clinical studies. The results presented below showed that devices satisfy the diagnostic test performance requirements. Sensitivity, assay precision, linearity, interfering substances, interfering antibodies, cross-reactivity, spiking recovery, dilution recovery, hook effect, and reference interval were assessed according to corresponding NCCLS guidelines EP5-A (http://www.zxyjhjy.com/upload/attached/file/20170406/20170406154608_8336.pdf).
6.1.1 Assay Precision
NR2 peptide ELISA assay precision results
Precision | Calibrator 1 | Calibrator 2 | Calibrator 3 | Negative control | Positive control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SD | Variance | CV | SD | Variance | CV | SD | Variance | CV | SD | Variance | CV | SD | Variance | CV | |
Repeatability (within-run) | 0.045 | 0.002 | 3.80 | 0.074 | 0.005 | 3.37 | 0.098 | 0.010 | 2.70 | 0.019 | 0.002 | 4.14 | 0.104 | 0.011 | 3.17 |
Between-run, within day | 0.018 | 0.000 | 1.55 | 0.072 | 0.005 | 3.31 | 0.106 | 0.011 | 2.92 | 0.008 | 0.001 | 1.73 | 0.051 | 0.003 | 1.55 |
Between-day | 0.100 | 0.010 | 8.45 | 0.016 | 0.000 | 0.72 | 0.040 | 0.002 | 1.11 | 0.009 | 0.000 | 2.00 | 0.047 | 0.002 | 1.43 |
Within device | 0.111 | 0.012 | 9.39 | 0.105 | 0.011 | 4.78 | 0.150 | 0.022 | 4.13 | 0.022 | 0.001 | 4.91 | 0.125 | 0.016 | 3.81 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


