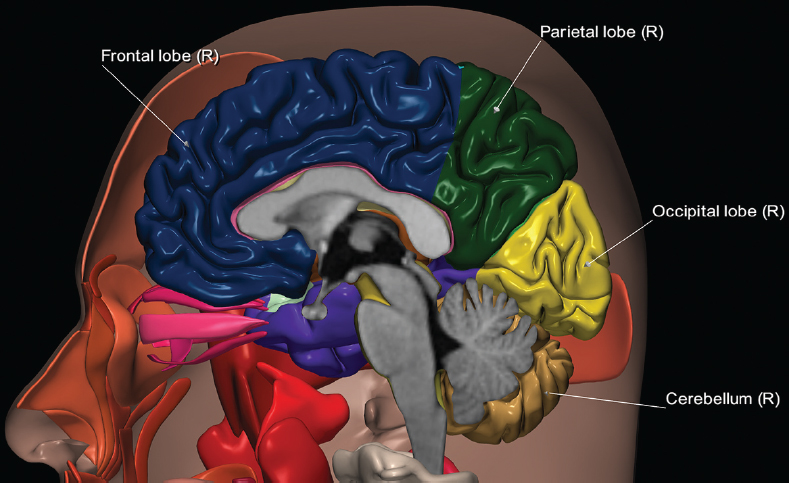
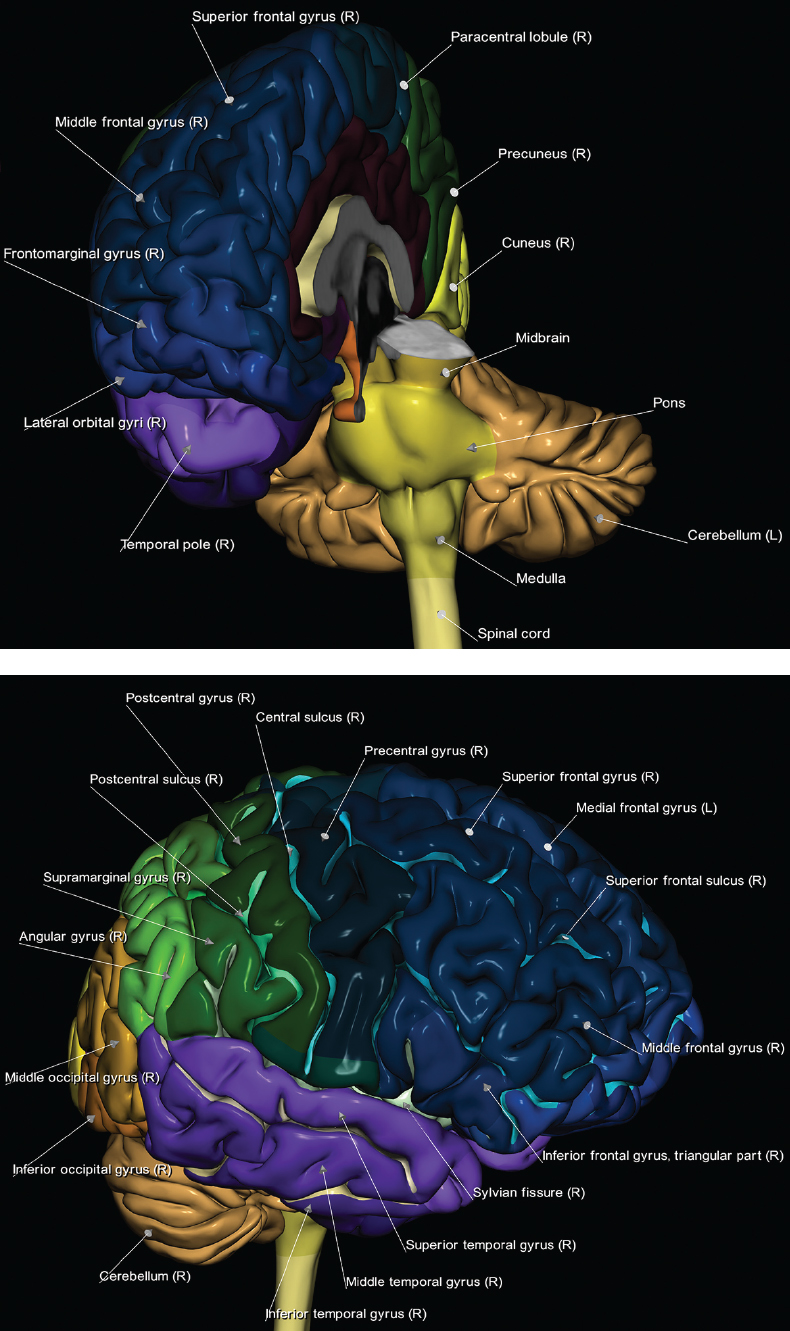
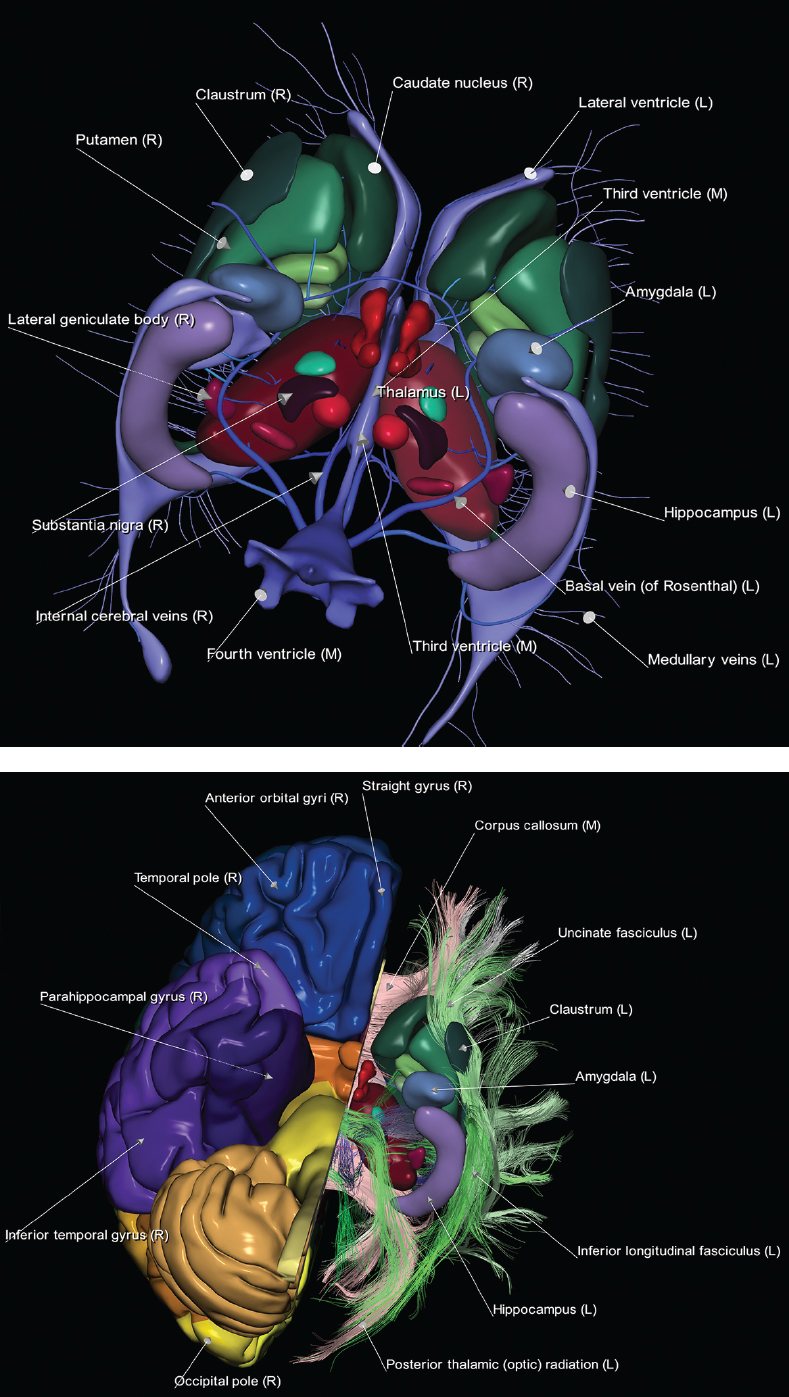
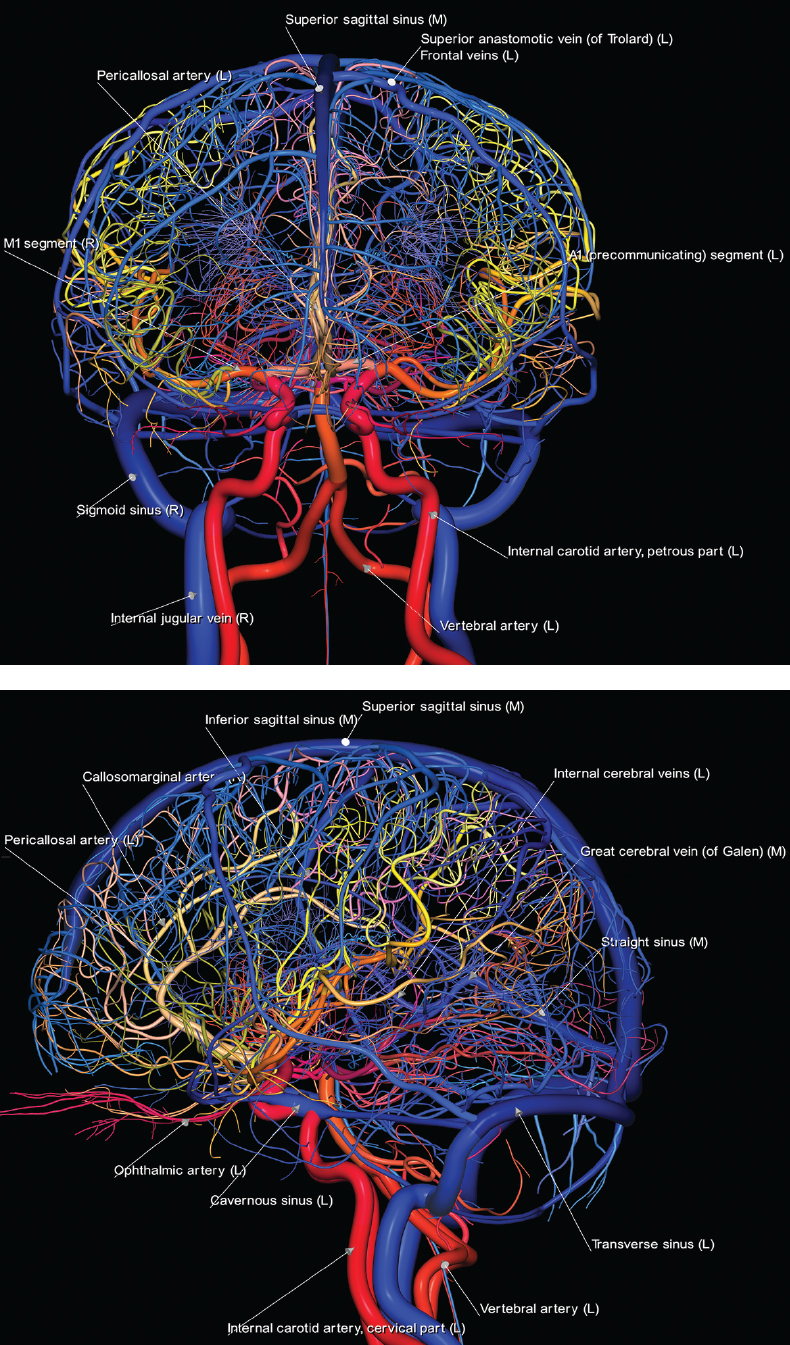
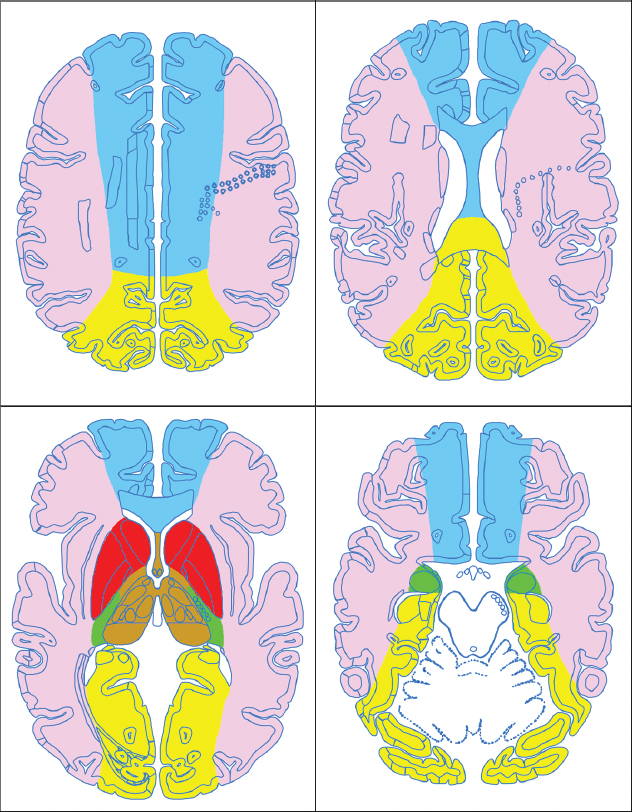
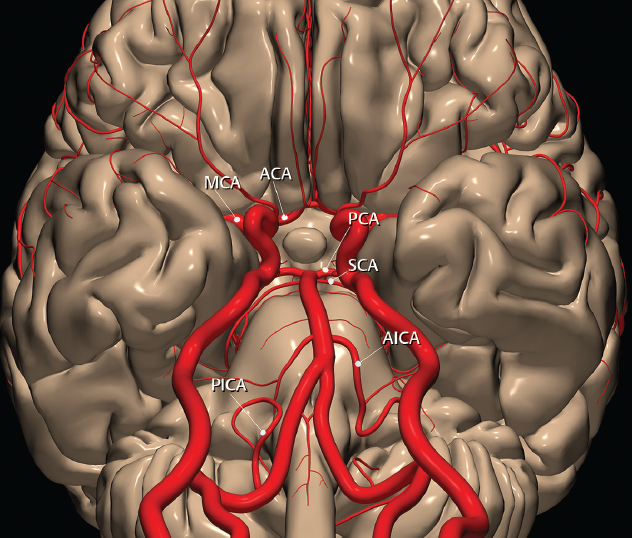
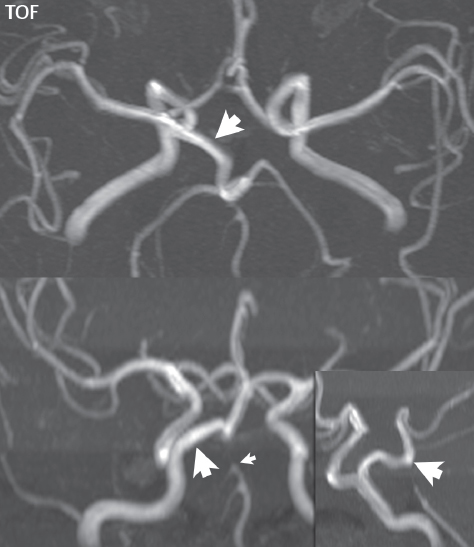
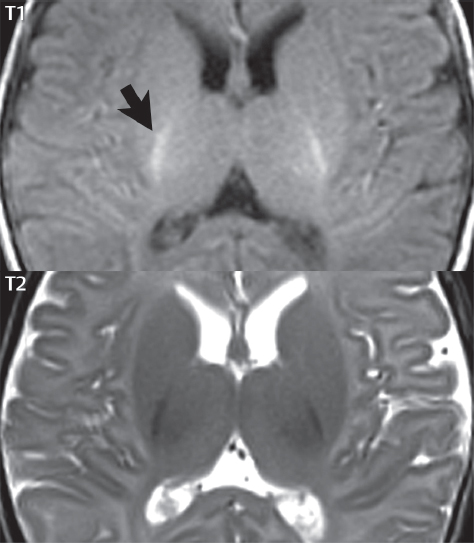
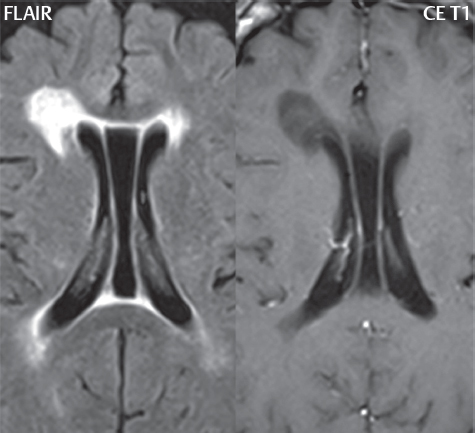
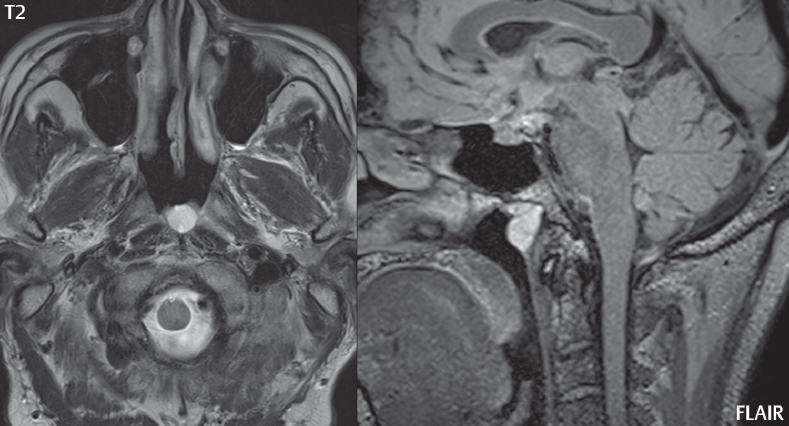
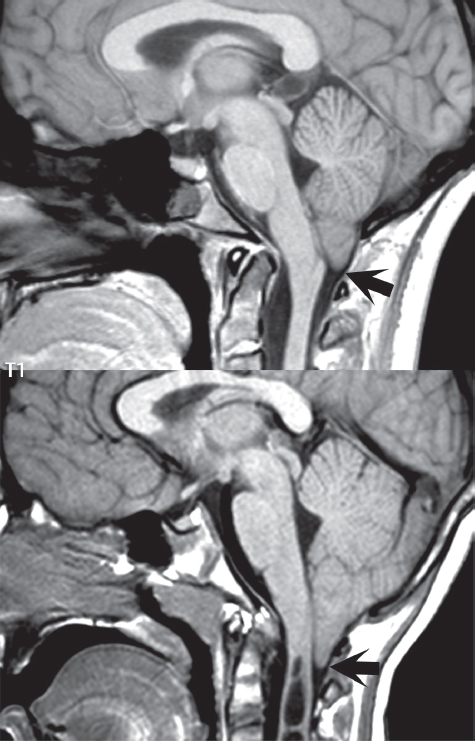
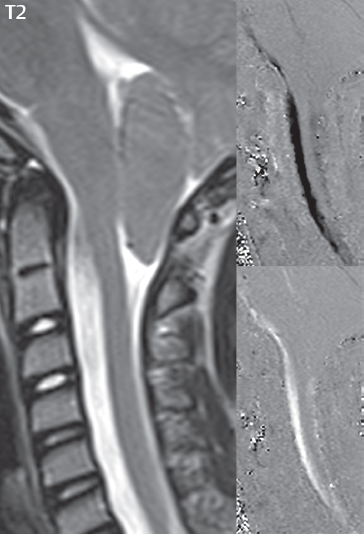
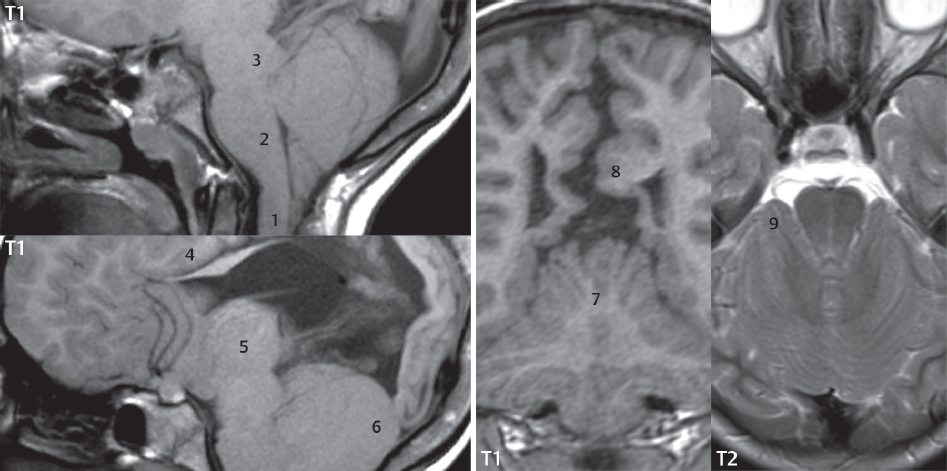
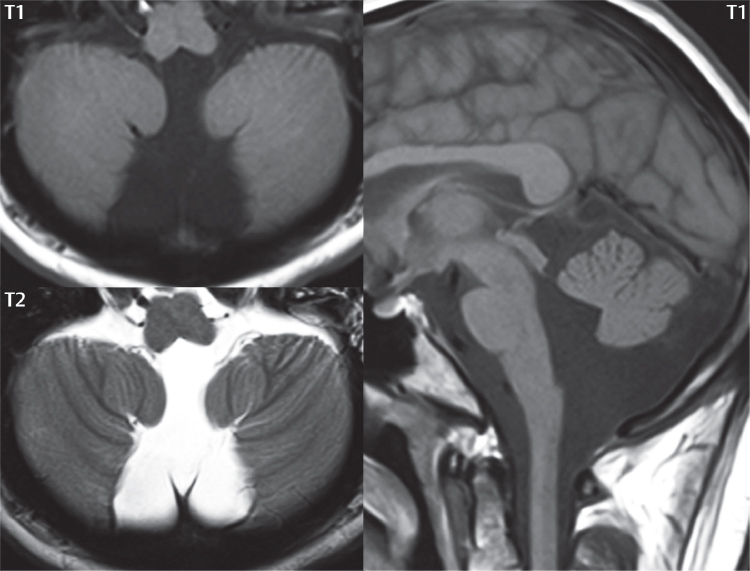
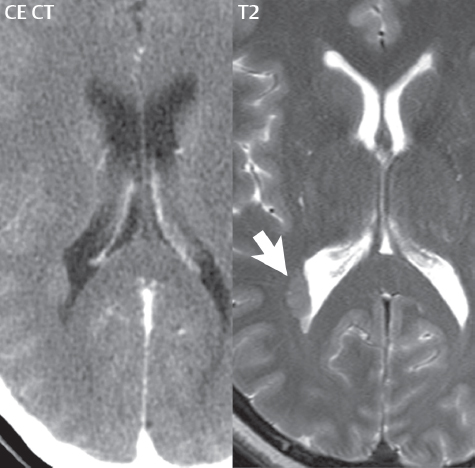
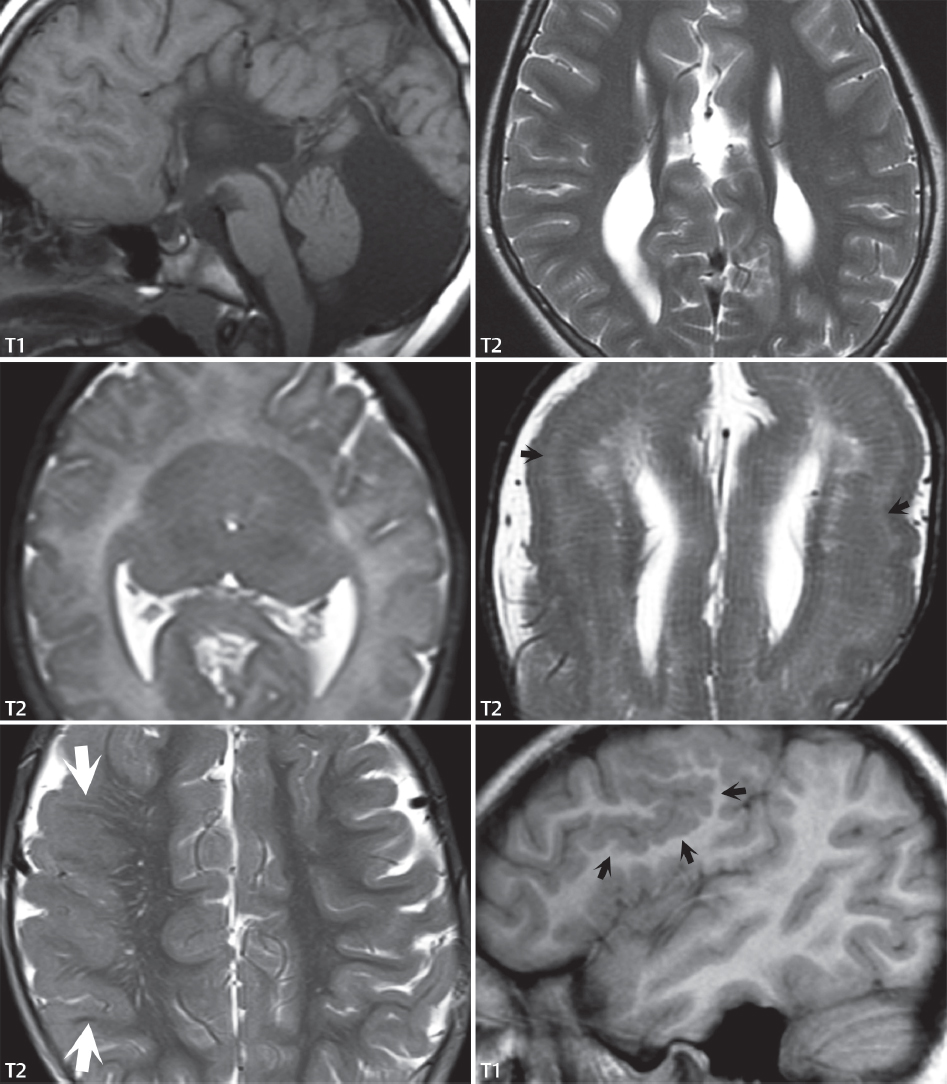
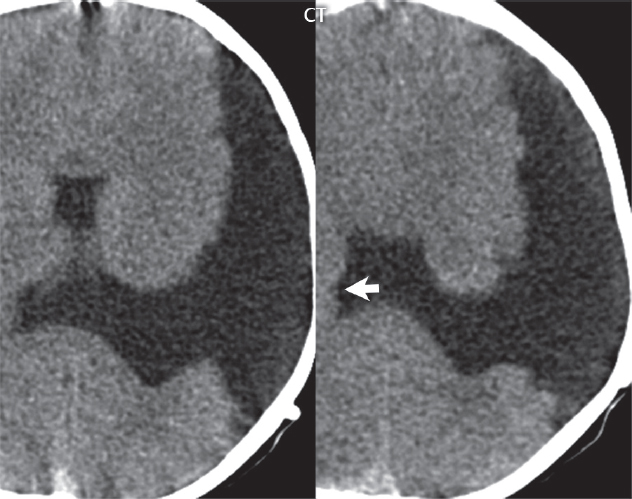
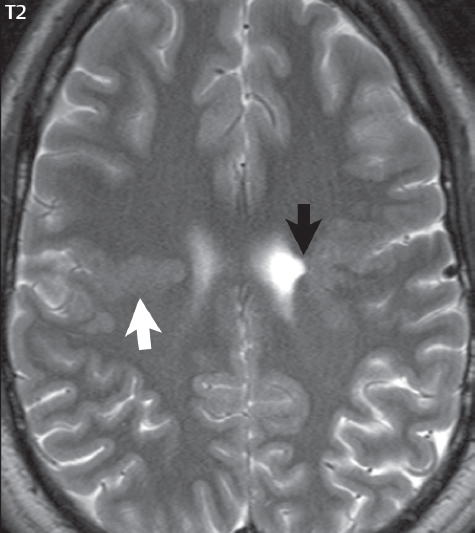
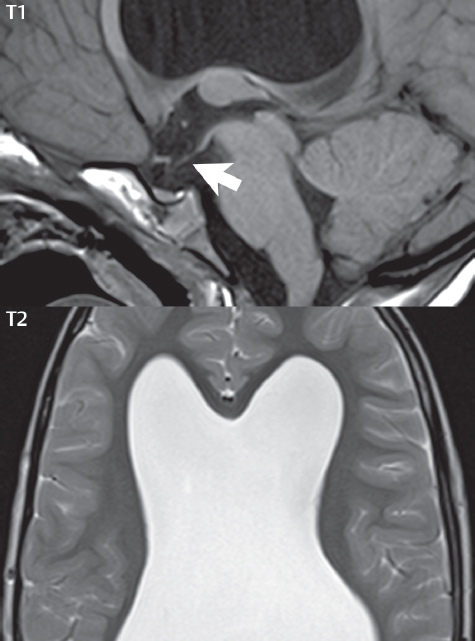
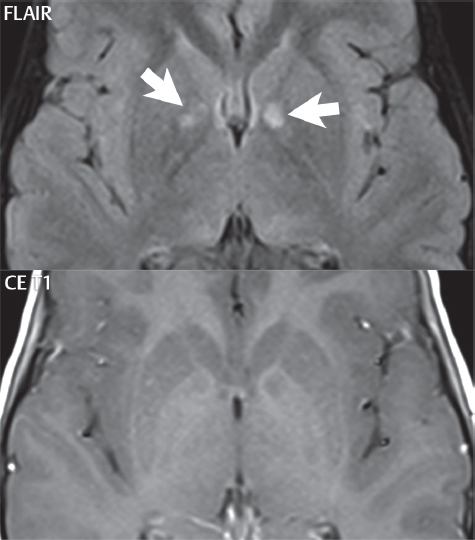
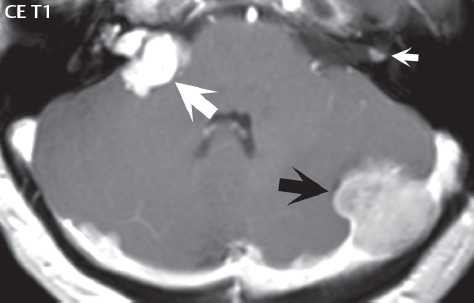
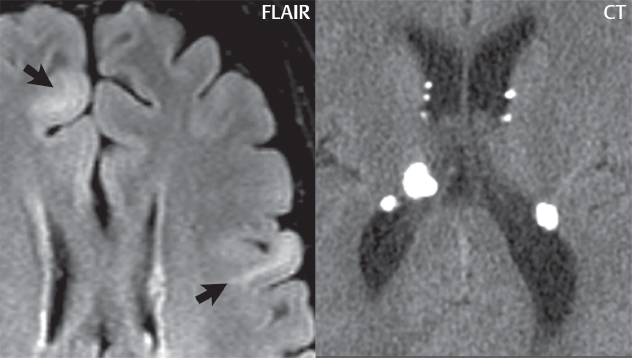
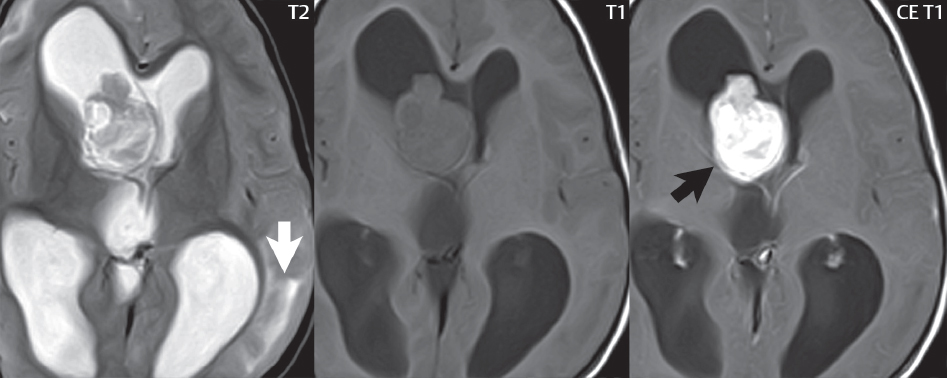
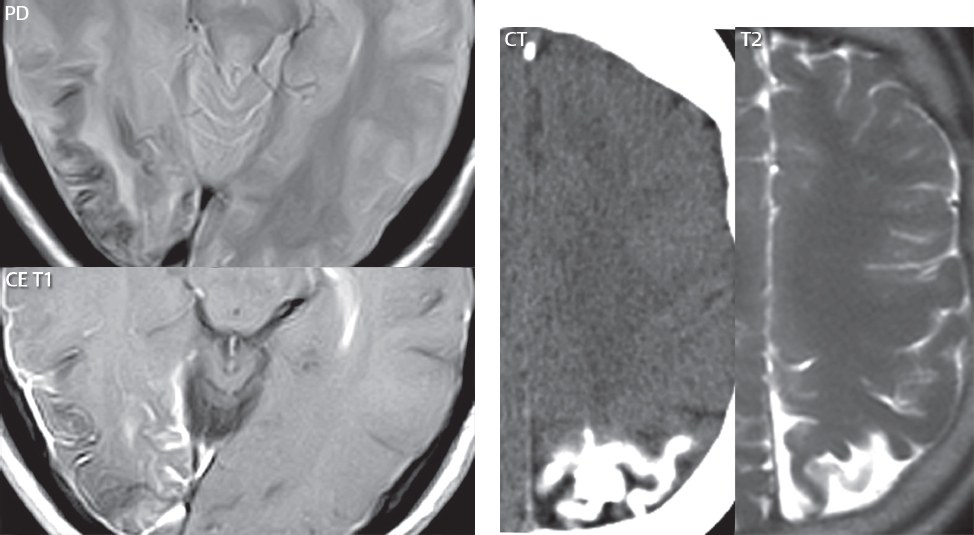
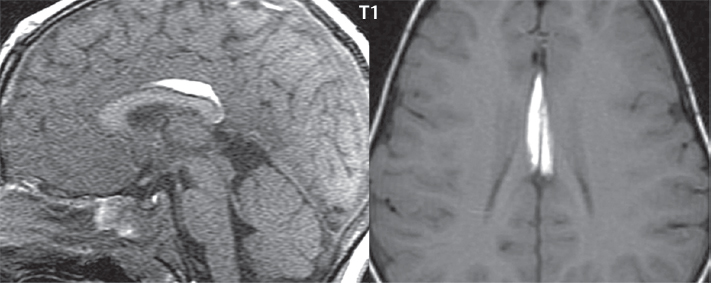
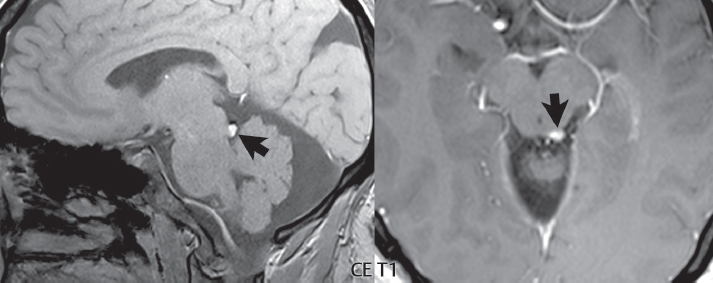
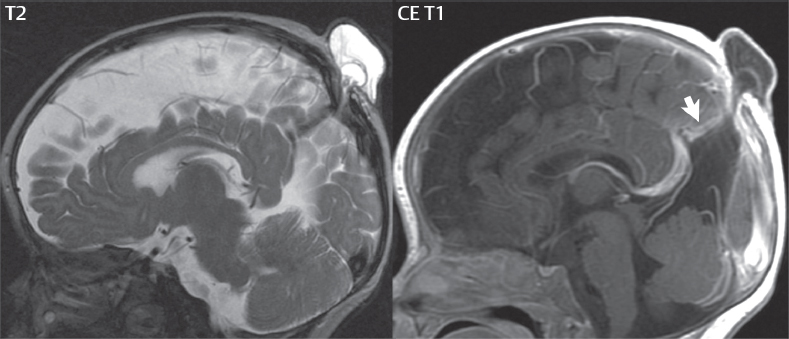
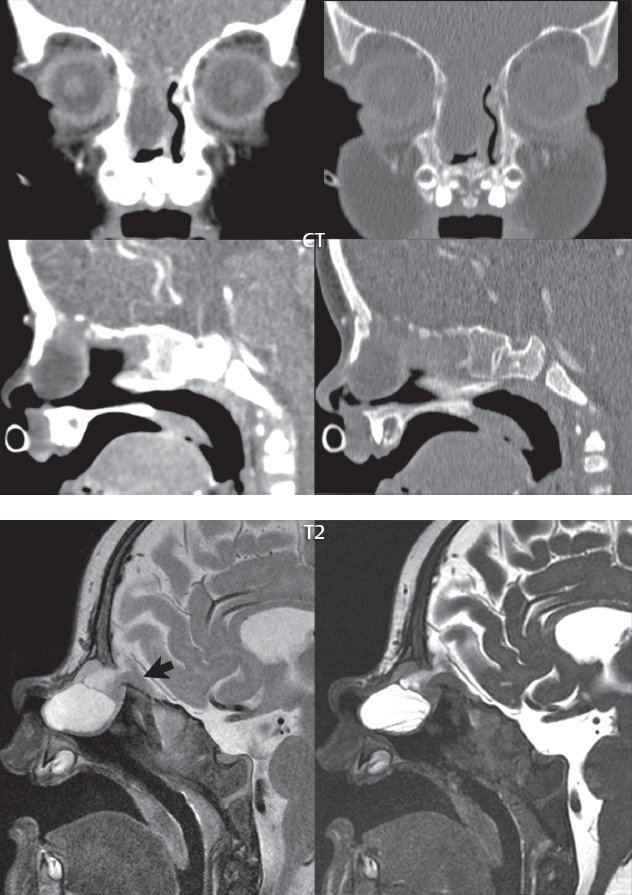
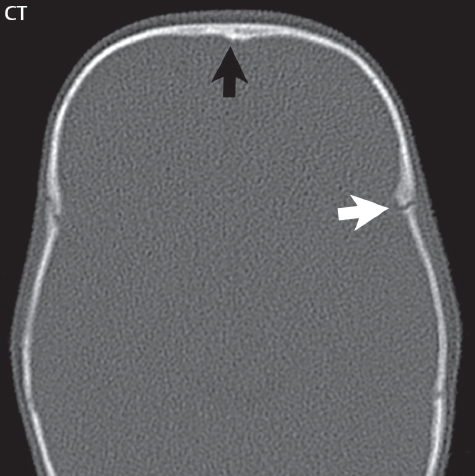
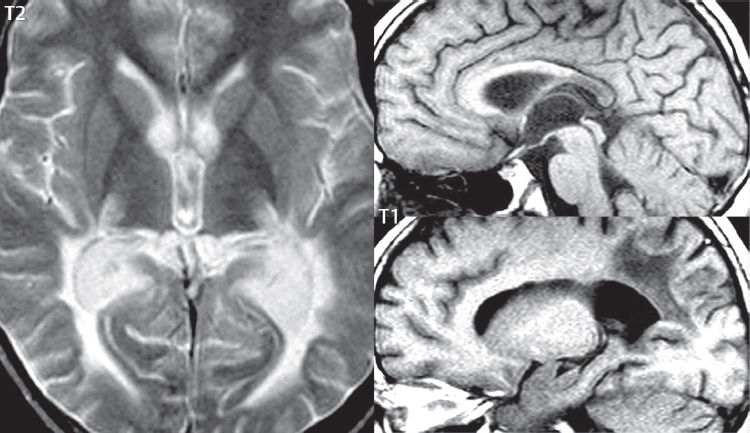
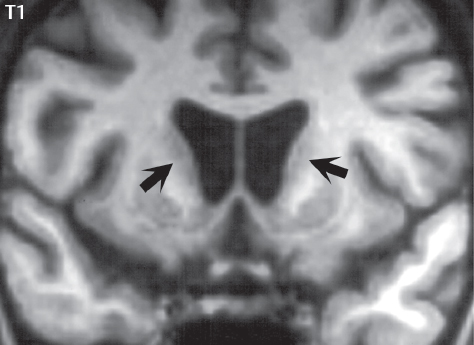
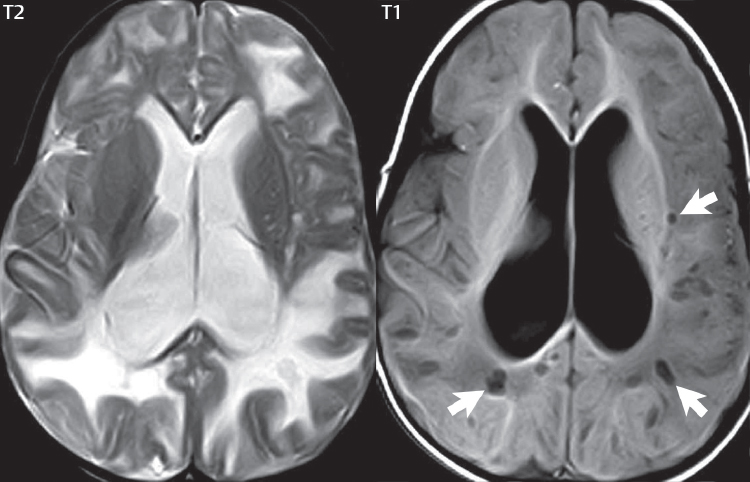
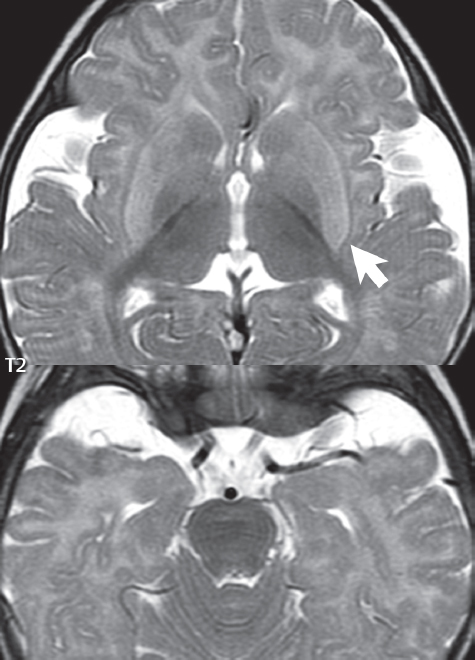
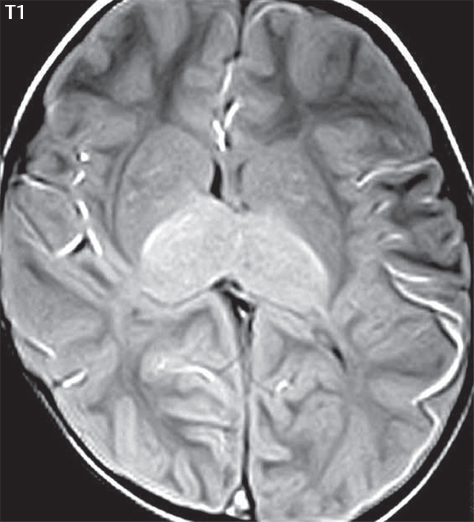
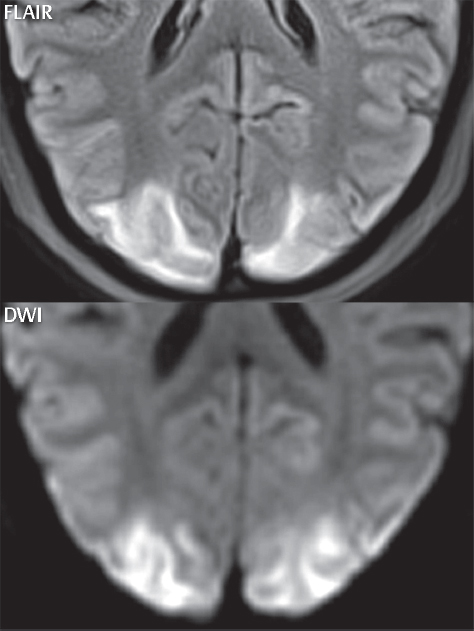
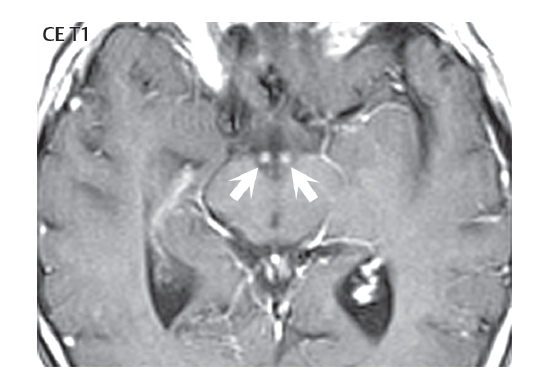
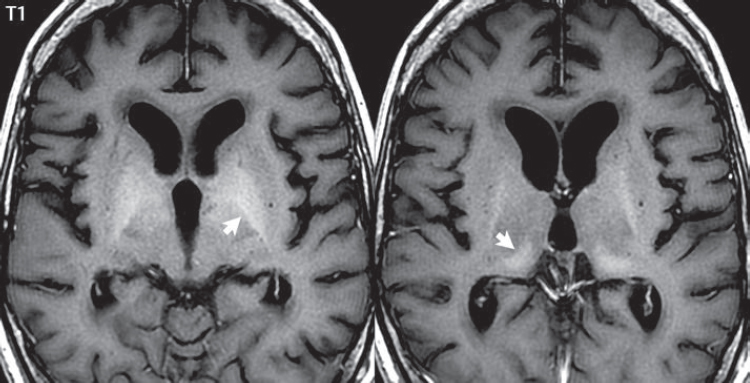
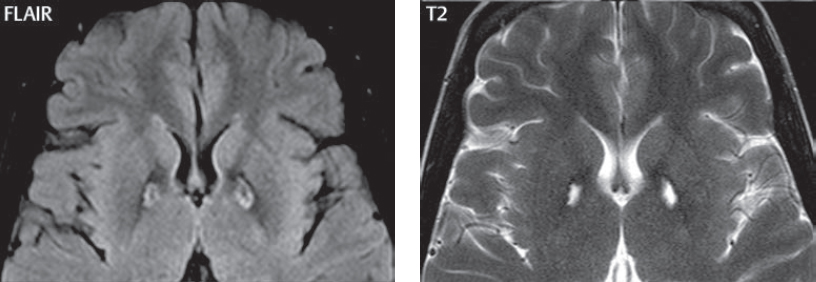
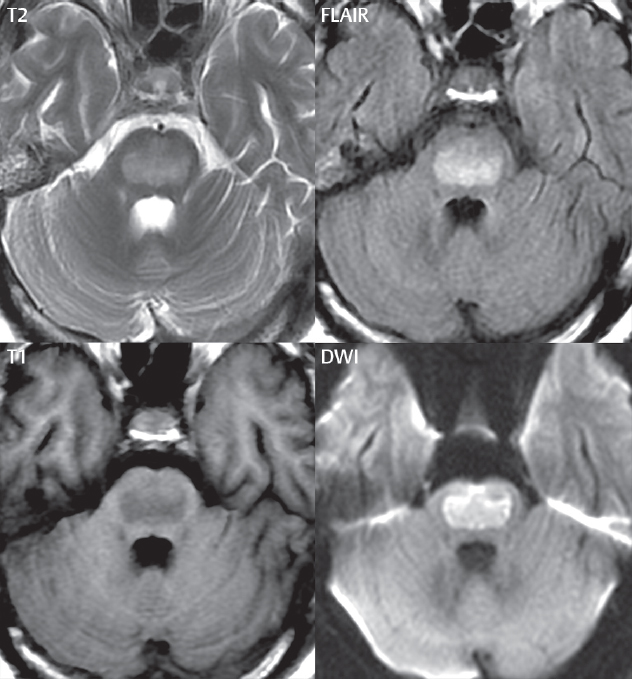
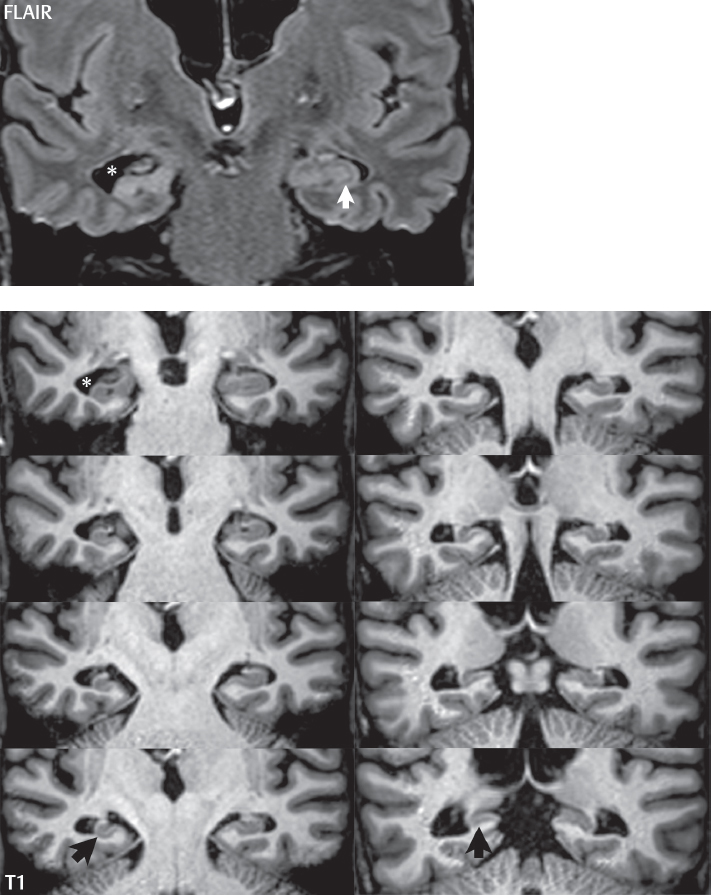
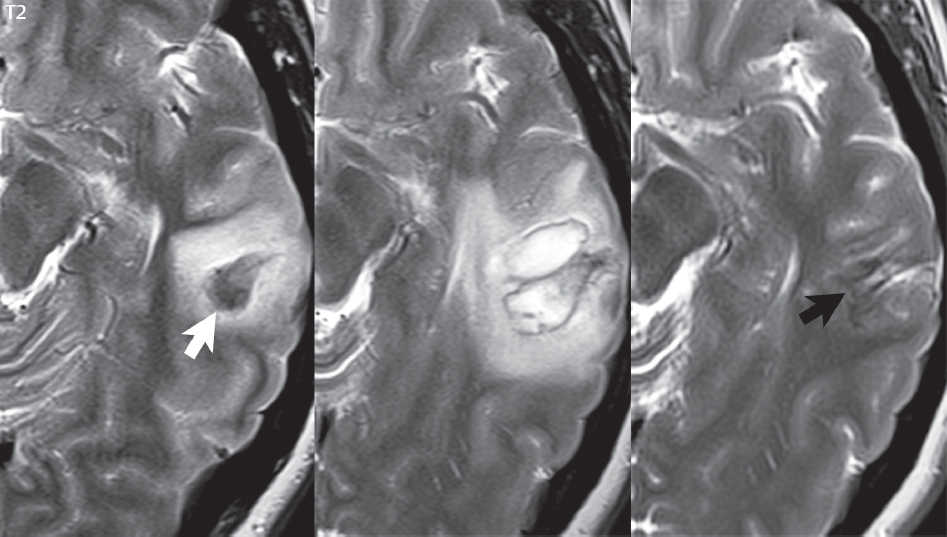
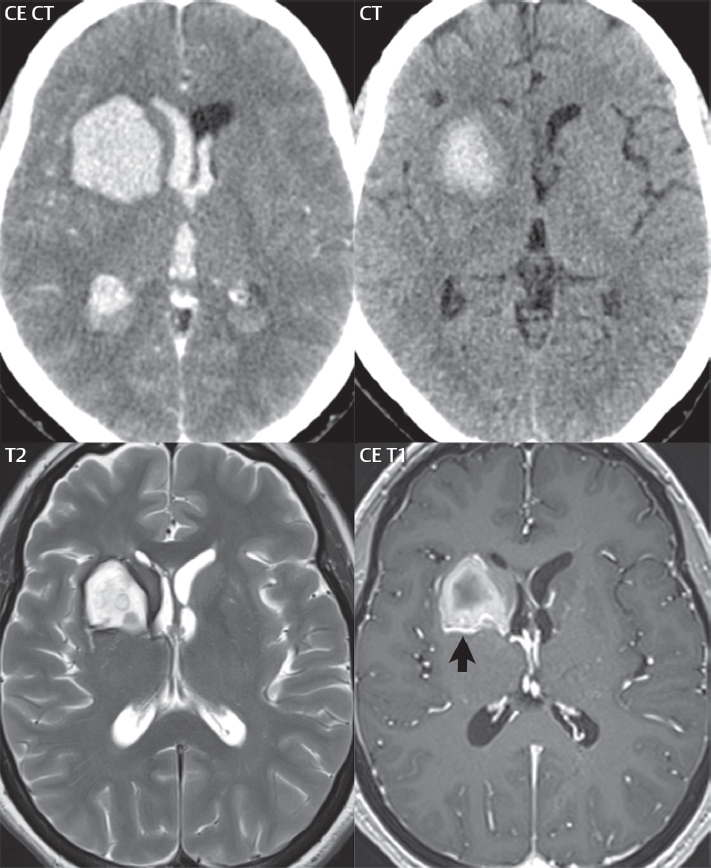
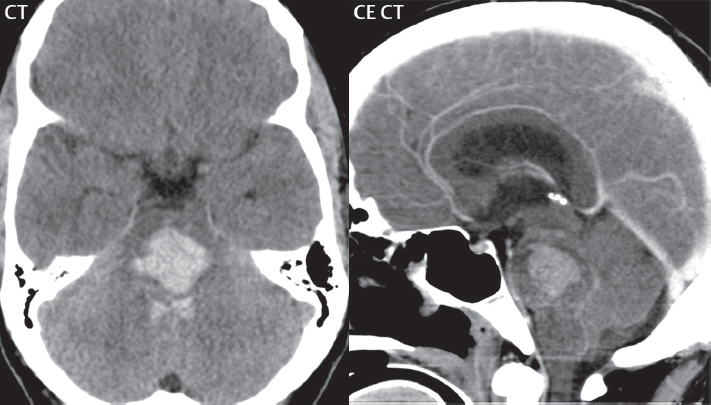
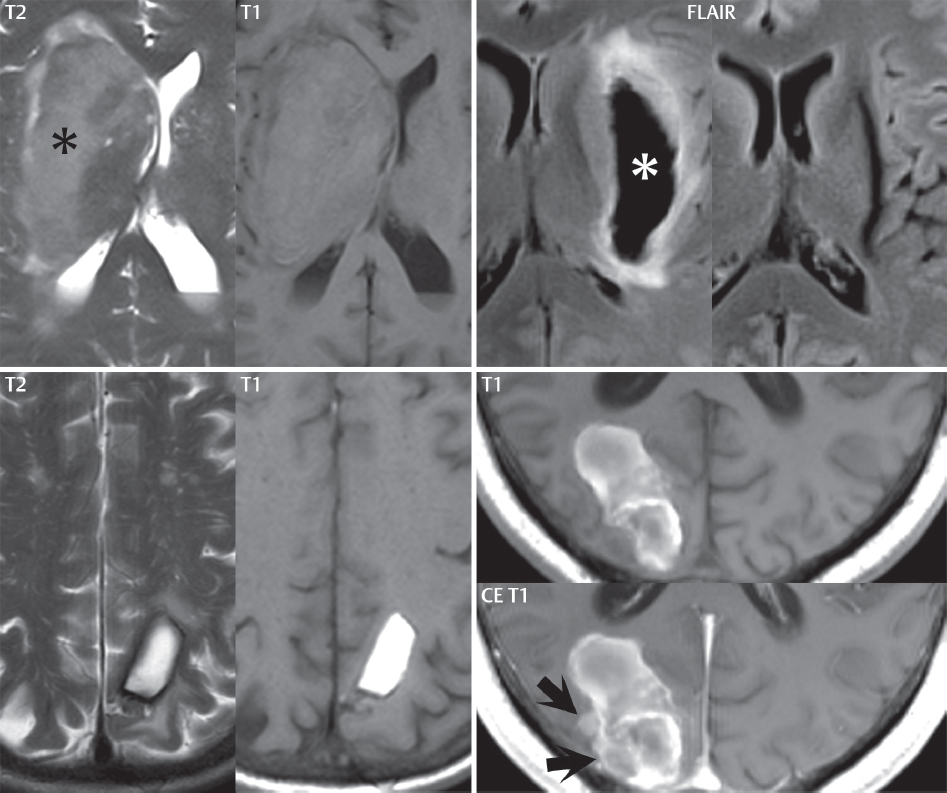
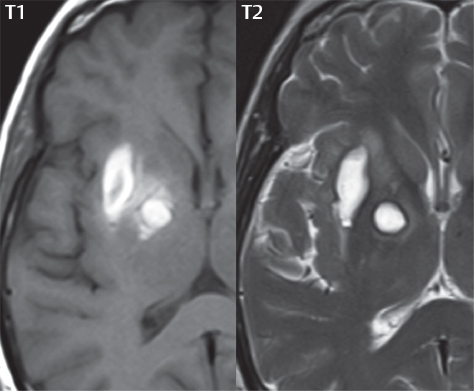
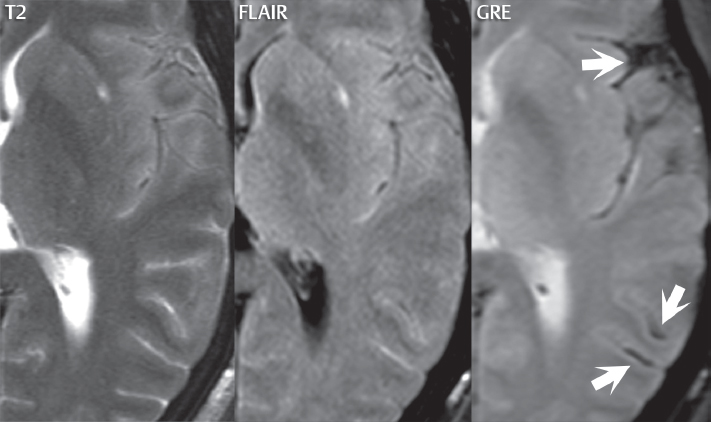
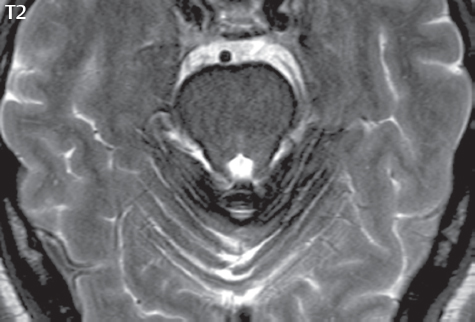
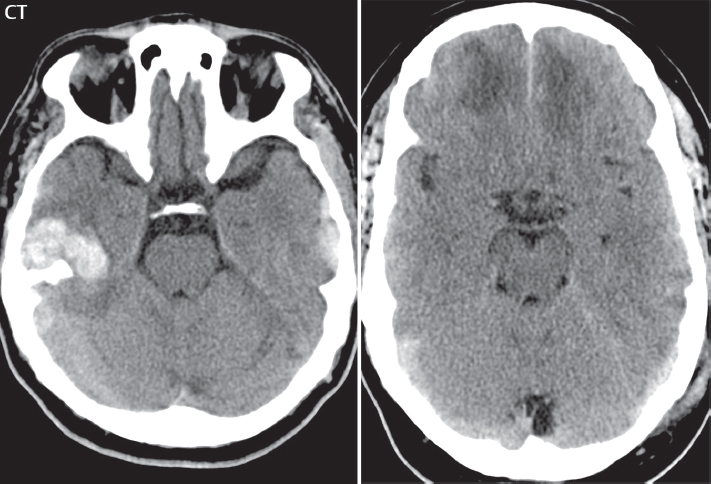
1 Brain The frontal lobe is demarcated posteriorly from the parietal lobe by the central sulcus (Fig. 1.1). On axial magnetic resonance (MR) images near the vertex, the central sulcus is readily identified. It is the major sulcus just behind the “L,” the intersection of two sulci formed in part by the superior frontal sulcus. The precentral gyrus lies just anterior to the central sulcus and the postcentral gyrus just posteriorly. As a generalization, the primary motor area (Brodmann area 4) is located in the precentral gyrus, and the primary somatesthetic (body’s sensations) area (Brodmann areas 1, 2, and 3) in the postcentral gyrus. The parietal lobe is demarcated from the occipital lobe posteriorly by the parieto-occipital sulcus (fissure). The anatomy of the nuclei and white matter tracts is beyond the scope of this book, but see Fig. 1.2. The reader is referred to the many computer-based atlases, including The Human Brain in 1969 Pieces by Wieslaw Nowinski. The pituitary gland is divided into anterior and posterior lobes. The anterior pituitary is referred to as the adenohypophysis and the posterior pituitary as the neurohypophysis. The normal pituitary is less than 10 mm in height, and demonstrates intense enhancement following intravenous contrast administration, due to the lack of a blood–brain barrier. A common variant in appearance of the pituitary is a slight upward convex superior margin, which can be seen in young women. On T1-weighted scans, the posterior pituitary is seen to be hyperintense in up to half of the normal patient population, a finding that is more common in younger patients (but not in the elderly). The internal auditory canal (IAC) is a bony foramen within the petrous portion of the temporal bone. It contains the 7th (facial) and 8th (vestibulocochlear) nerve complexes. The 7th nerve lies in the anterior superior quadrant of the IAC, when viewed in cross-section, and runs laterally to the geniculate ganglion. The cochlear division of the 8th nerve lies in the anterior inferior quadrant. The superior and inferior vestibular nerves, also divisions of the 8th nerve, which supply information concerning equilibrium, lie in the superior and inferior posterior quadrants. As visualized in the axial plane, the cochlea is anterior and the vestibule posterior. There are three semicircular canals: the lateral (which has a horizontal orientation), superior, and posterior. The fluid (endolymph) within the cochlea, vestibule, and semicircular canals is normally isointense to cerebrospinal fluid (CSF) on MR. Three major arteries supply the cerebral hemispheres (Fig. 1.3). The anterior cerebral artery (ACA) supplies the anterior two-thirds of the medial cerebral surface and 1 cm of superior medial brain over the convexity. The recurrent artery of Heubner, which originates from the A1 or A2 segment of the ACA, supplies the caudate head, anterior limb of the internal capsule, and part of the putamen. The posterior limb of the internal capsule, portions of the thalamus, the caudate, the globus pallidus, and the cerebral peduncle are supplied by the anterior choroidal artery, which arises from the supraclinoid internal carotid artery. The middle cerebral artery (MCA) supplies the lateral portion of the cerebral hemispheres, the insula, and the anterior and lateral temporal lobes. The lenticulostriate arteries, which originate from the M1 segment of the MCA, supply the lentiform nucleus (globus pallidus and putamen) and the anterior limb of the internal capsule. The posterior cerebral artery (PCA) supplies the occipital lobe and the medial temporal lobe. The thalamoperforating and thalamogeniculate branches supply the medial portion of the thalami and the walls of the third ventricle. These small perforating branches arise from the P1 segment of the PCA with similar branches arising from the posterior communicating artery (Fig. 1.4). Fig. 1.1 Normal lobar and gyral anatomy. Important landmarks include the central sulcus, which separates the frontal lobe anteriorly from the parietal lobe posteriorly, and the sylvian fissure (the lateral sulcus), which divides the frontal and parietal lobes above from the temporal lobe below. The parietal and occipital lobes are separated by the parieto-occipital sulcus. (Courtesy of Wieslaw Nowinski, DSc, PhD.) Fig. 1.2 Brain nuclei and white matter tracts, normal anatomy. Most relevant to MR interpretation are the locations of the caudate nucleus, putamen, globus pallidus (light green, immediately medial to the putamen and not labeled), hippocampus, and thalamus. Note also the location of the optic radiations. (Courtesy of Wieslaw Nowinski, DSc, PhD.) Three major but smaller vessels supply the cerebellum (Fig. 1.5). The largest is the posterior inferior cerebellar artery (PICA), which supplies the tonsil, the inferior vermis, and the inferior cerebellum (with the exception of its most anterior extent). The anterior inferior cerebellar artery (AICA) supplies the anterior inferior portion of the cerebellum and is the smallest of the three vessels. It is commonly stated that the distribution of AICA is in continuum with PICA, with at times the distribution slightly larger or smaller. The superior cerebellar artery supplies the superior half of the cerebellum. The circle of Willis is complete in only one quarter of the population. Variants include the following. A fetal origin of the posterior cerebral artery, with its origin from the internal carotid artery instead of the basilar artery, is seen in about one in five patients. The P1 segment of the posterior cerebral artery, which is the portion from the tip of the basilar artery to the junction with the posterior communicating artery (PCOM), is usually also hypoplastic in this circumstance. The PCOM is hypoplastic in one-third of patients. The anterior communicating artery (ACOM), which connects the two anterior cerebral arteries, is hypoplastic in 15%. The A1 segment of the anterior cerebral artery, which begins at the carotid terminus and continues to the juncture with the ACOM, is hypoplastic in 10%. Fig. 1.4 Supratentorial arterial territories. Axial diagrams of the brain at four levels depict the major arterial territories of the supratentorial region, specifically the anterior cerebral artery (blue), middle cerebral artery (pink), and posterior cerebral artery (yellow) territories. In red is the vascular territory supplied by the penetrating branches of the middle cerebral artery (the lenticulostriate arteries). In brown is the territory supplied by the penetrating branches of the posterior cerebral arteries (the posterior thalamoperforators) and posterior communicating arteries (the anterior thalamoperforators). In green is the territory supplied by the anterior choroidal artery, which supplies amongst other structures the posterior limb of the internal capsule, the optic tract, and the hippocampus and amygdala. (Courtesy of Wieslaw Nowinski, DSc, PhD.) Fig. 1.5 Arterial supply of the posterior circulation, visualized on an anatomic drawing of the base of the brain. The posterior inferior cerebellar artery (PICA) originates from the vertebral artery. The two vertebral arteries join to form the basilar artery, with the major paired branches (in order from caudal to cranial) being the anterior inferior cerebellar artery (AICA), the superior cerebellar artery (SCA), and the posterior cerebral artery (PCA)—the latter marking the termination of the basilar artery. (Courtesy of Wieslaw Nowinski, DSc, PhD.) The external carotid artery is the smaller of the two terminal branches of the common carotid artery. It arises anterior and medial to the internal carotid artery, then courses posterior laterally. There are many muscular branches, with the early branching of the external carotid artery allowing rapid recognition of this vessel in distinction to the internal carotid artery. The internal carotid artery was traditionally divided into four major segments: the cervical, the petrous (horizontal), the cavernous (juxtasellar), and the intracranial (supraclinoid) portions. Today, there are seven recognized segments (C1 to C7): the cervical, petrous, lacerum, cavernous, clinoid, ophthalmic, and communicating (terminal) segments. At its origin, the internal carotid artery is somewhat dilated, forming the carotid bulb. The petrous segment, C2, of the internal carotid artery has three sections: the ascending (vertical), the genu (bend), and the horizontal portions. The lacerum segment, C3, is still extradural. The cavernous segment, C4, is surrounded by the cavernous sinus. The meningohypophyseal artery arises from C4. The clinoid segment, C5, is very short, and begins after the artery exits from the cavernous sinus. C5 extends from the proximal dural ring to the distal dural ring. C6, the ophthalmic segment, extends from the distal dural ring (with this portion of the internal carotid artery thus considered intradural) to the origin of the PCOM. The ophthalmic artery arises from C6. C7 is that segment of the artery extending from the origin of the posterior communicating artery to the carotid terminus, where the vessel divides into the anterior and middle cerebral arteries. C6 and C7 together constitute the supraclinoid internal carotid artery. There are many extracranial–intracranial vascular anastomoses. Two of these involve the ophthalmic artery. There are also multiple internal carotid–vertebral artery anastomoses, which represent persistent embryonic circulatory patterns. One of these is seen not uncommonly, as a normal variant, and is the persistent trigeminal artery (Fig. 1.6). Pial–leptomeningeal anastomoses are also present, and are an important potential source of collateral blood flow in occlusive vascular disease. Fig. 1.6 Persistent trigeminal artery. Three projections from a 3D time-of-flight MRA of the circle of Willis are presented. The proximal basilar artery is small, and terminates in its mid-section (small arrow). The distal basilar artery is supplied from the right internal carotid artery, via a persistent embryonic connection (large arrow). Regarding the central venous anatomy of the brain, the paired internal cerebral veins and basal veins of Rosenthal join to form the vein of Galen. The latter is then joined by the inferior sagittal sinus, which lies along the free edge of the falx, to form the straight sinus, which drains to the confluence of the sinuses (torcular herophili). Superficial cerebral veins over the convexity join to form the superior sagittal sinus, which lies along the midline, which then drains to the confluence of the sinuses. Flow continues via the transverse sinuses, which are often asymmetric (with the right usually dominant), to the sigmoid sinus, the jugular bulb, and then the internal jugular vein. There are three large named superficial veins. There is the superficial middle cerebral vein, which lies in the Sylvian fissure and drains into the cavernous or sphenoparietal sinus. The vein of Trolard joins the superior sagittal sinus and the superficial middle cerebral vein. The vein of Labbe joins the transverse sinus and the superficial middle cerebral vein. The venous system is currently best demonstrated (when considering computed tomography [CT] and MR) on two-dimensional (2D) time of flight (TOF) MR angiography. Myelination begins in the brainstem, progresses to the cerebellum and cerebrum, with the order of myelination from central to peripheral, inferior to superior, and posterior to anterior. T1-weighted images are particularly useful to assess myelination in the first 9 months of life. With normal myelination, on T1-weighted images, white matter becomes higher in signal intensity. T2-weighted images are more useful to assess myelination after 6 months of age. However, it is important to note that longer repetition times (TRs) are required to evaluate the brain under 2 years of age (with T2-weighted scans), when compared to the adult. On T2-weighted images, with normal myelination, white matter becomes lower in signal intensity. This is due to the lower water content as myelin matures. Myelination is quite specific for age from the newborn to 2 years of life; however, for simplification, the normal appearance is discussed at only four time frames. The dorsal pons, superior and inferior cerebellar peduncles, posterior limb of the internal capsule, and ventral lateral thalamus will demonstrate partial myelination, best seen on T1-weighted scans, in the newborn (Fig. 1.7). The corpus callosum at this time is not myelinated, and will also appear thin. At 6 months of age, on T1-weighted scans, the white matter of the cerebellum, the anterior limb and genu of the internal capsule, the white matter of the occipital lobe, and the posterior centrum semiovale will all appear normally myelinated with high signal intensity. The corpus callosum at this age will be partially myelinated, but will also still appear to be thin. At 6 months of age, on T2-weighted scans, only the posterior limb of the internal capsule will demonstrate low signal intensity, indicative of myelination. Fig. 1.7 Normal myelination in a newborn. At birth, the brain is principally not myelinated, with the appearance on MR strikingly different on both T1- and T2-weighted images when compared to the adult brain. Gray matter is predominantly of slightly higher signal intensity when compared to white matter on a T1-weighted scan in the newborn, with a principal indicator of early myelination being high signal intensity in the posterior limb of the internal capsule (black arrow). At 12 months of age, on a T1-weighted scan, there will be a near adult pattern of myelination, specifically seen both in the deep and peripheral white matter. This pattern is reached by 9 months of age. On a T2-weighted scan, the deep white matter, specifically the internal capsule, corpus callosum, and corona radiata, will appear mature, with low signal intensity. Myelination is not yet complete at this age, as depicted on the T2-weighted scan, in the white matter of the frontal, temporal, parietal, and occipital lobes, together with the more peripheral subcortical white matter. These areas will still appear isointense to gray matter, not the low signal intensity (SI) on a T2-weighted scan indicative of mature myelination. At 2 years of age, the deep and superficial white matter of the frontal, temporal, parietal, and occipital lobes will appear low SI on T2-weighted scans, similar to the adult pattern. The SI of these areas will however not be as low as the internal capsule, with that not occurring until 3 years of age. The deep white matter of the parietal lobes, surrounding the ventricular trigones, is the last area to completely myelinate (the so-called zone of terminal myelination). Mild hyperintensity on T2-weighted scans in this region may persist up to 10 years of age. The septum pellucidum is a thin translucent plate, consisting of two laminae (leaves), that lies between the lateral ventricles. It extends from the corpus callosum superiorly to the fornix inferiorly. A cavum septum pellucidi is a common variant in which the two leaves are separated. This is a normal embryologic space, and is seen in all fetuses and premature infants. By 3 to 6 months of age only 15% of infants have a cavum septum pellucidi. The separation of leaves can persist into adulthood and as such is considered a normal variant. A cavum septum vergae is also a normal embryologic space, like a cavum septum pellucidi but less common. It is essentially a posterior extension of a cavum septum pellucidi. It is seen as a midline cavity posterior to the columns of the fornix, which ends at the splenium of the corpus callosum. The internal cerebral veins lie inferiorly. When present in an adult, it is also considered to be a normal variant. The most common presentation is together with a cavum septum pellucidi, and in this instance the term cavum septum pellucidi et vergae (Fig. 1.8) is used. A cavum velum interpositum is a much less common variant, describing a cyst between the fornices superiorly and the roof of the third ventricle inferiorly. The internal cerebral veins lie within a cavum velum interpositum. An absent septum pellucidum is rare, and almost always signifies major neurologic disease. It is associated with many congenital malformations, including septo-optic dysplasia. An absent septum pellucidum can be an acquired abnormality, due to chronic hydrocephalus. Fig. 1.8 Cavum septum pellucidi et vergae. In this patient with multiple sclerosis, and a prominent nonenhancing (chronic) right frontal plaque, the septum pellucidi are separated anteriorly, an extremely common normal variant. The division continues in this patient posteriorly, between the fornices, leading to a cavum septum pellucidi (anteriorly) and a cavum vergae (posteriorly). The glomus portion of the choroid plexus, contained in the atria of the lateral ventricles, is the most frequent portion of the choroid plexus to calcify. Calcifications are usually globular and bilateral. Calcification and/or ossification is also commonly seen by CT in the falx (and less often in the pineal gland). These are all typically incidental findings. Because only dense, prominent calcification appears as low signal intensity on MR, calcification per se is less commonly visualized on MR. If the calcification is of sufficient size, it will however be seen on conventional MR. Ossification of the falx is well seen on MR due to visualization of the high signal intensity fat therein. Calcification and iron deposition are both dystrophic processes, and can occur together. Thus, sometimes what is seen as calcification on CT can be visualized as an abnormality on MR, but actually represents iron (hemosiderin) deposition. A pineal cyst is a common normal variant, and almost always asymptomatic. It is best visualized on thin section sagittal MR images. These are ovoid in shape, smoothly marginated, with a very thin wall, and rarely greater than 15 mm in diameter. The fluid therein will be homogeneous, with near CSF SI. On FLAIR, the cyst fluid will have slight high signal intensity. A thin uniform rim of contrast enhancement is common. Some large, but still asymptomatic, pineal cysts appear to have slight mass effect upon the adjacent colliculi. Choroidal fissure cysts occur in the medial temporal lobe near the choroidal fissure. Their extra-axial location is easily confirmed on coronal images. Choroidal fissure cysts have a characteristic spindle shape when viewed in the sagittal plane. The terms Virchow-Robin space and perivascular space are used interchangeably. This is a normal CSF space surrounding the perforating arteries entering the brain, and represents an invagination of the subarachnoid space. In the elderly, perivascular spaces may be more prominent. There are three common locations in which dilated perivascular spaces are seen. The first location is within the inferior third of the lentiform nucleus. In this instance the dilated spaces lie adjacent to the anterior commissure, following the course of the lenticulostriate arteries. Although usually less than 5 mm in diameter, larger dilated perivascular spaces can be seen in this location. These should be isodense on CT and isointense on MR to CSF. Differentiation can be difficult at times from chronic lacunar infarcts, with the latter the more common finding superiorly in the lentiform nucleus. The second common location for dilated perivascular spaces is within the white matter of the centrum semiovale. These follow the course of nutrient arteries, which lie along the white matter radiations. Thus, depending upon orientation relative to the slice, they may be seen either in cross-section or in plane, the latter as small radial structures. The third site, which is less common than the other two, is in the cerebral peduncle (near the substantia nigra). Although bilateral lesions may be seen here, typically the dilated perivascular space on one side is much larger than the other. Arachnoid granulations are small focal areas of arachnoid that protrude through the dura into the venous sinuses of the brain. CSF exits from the subarachnoid space via arachnoid granulations and enters the bloodstream, in part due to the normal higher pressure of CSF. These granulations also function as one-way valves. As MR has improved in terms of image quality and spatial resolution, visualization of arachnoid granulations within the transverse sinuses is not unusual. These should not be confused for venous thrombi. A Tornwaldt cyst (Fig. 1.9) is a common finding on MR of the brain, and is considered to be an incidental (benign) lesion. These vary in size, but are typically small. Tornwaldt cysts are thought to arise from the notochordal remnant, and occur in the posterior superior nasopharynx along the midline. They can be high signal intensity on T1-weighted scans, reflecting protein content. Epidermoid cysts and small lipomas of the scalp are commonly encountered, incidentally. These entities have characteristic imaging appearances, on both CT and MR, and should be kept in mind when encountering a focal scalp lesion, due to their high incidence. The common small, painless lump under the skin seen incidentally on head CT is actually an epidermoid cyst, although often (incorrectly) termed “sebaceous cysts” by radiologists. The latter are much less common than epidermoid cysts, and affect the sebaceous glands, the oil glands in the skin. MR is the modality of choice for evaluation of all congenital malformations of the brain, with the exception of the craniosynostoses. In individual instances, some findings may be apparent on CT, with it thus being important to keep in mind the congenital malformations of the brain when interpreting CT. In a Chiari I malformation, there is displacement of the cerebellar tonsils > 5 mm below the level of the foramen magnum. This abnormality is not uncommon, and usually asymptomatic. On sagittal images the cerebellar tonsils are pointed or wedge-shaped (Fig. 1.10). The fourth ventricle will be in normal position. Symptoms occur when there is obstruction of CSF flow through the foramen magnum. If there is crowding at the level of the foramen magnum, CSF flow studies can be obtained on MR to determine if the flow is abnormal and thus likely to contribute to symptoms (most frequently headache) (Fig. 1.11). In symptomatic cases, there can be dilatation of the central canal of the spinal cord, specifically hydromyelia (more generally termed syringohydromyelia). Treatment is surgical, by suboccipital decompression with resection of the posterior arch of C1. A Chiari I malformation is to be distinguished from tonsillar ectopia, specifically mild inferior displacement of the cerebellar tonsils seen in asymptomatic normal individuals. In this entity, the tonsils retain their normal globular configuration. In most normal individuals, the tonsils lie above the level of the foramen magnum, but they may lie as far as 5 mm below and still be normal. Fig. 1.9 Tornwaldt cyst. Axial T2-weighted and sagittal FLAIR scans reveal a well-circumscribed, round cyst along the midline in the upper posterior nasopharynx. This finding is considered incidental, of no clinical consequence. The Chiari II malformation is a complex congenital brain anomaly, which involves principally the hindbrain (the medulla, pons, and cerebellum). Additional features involve the forebrain (the cerebral hemispheres, basal ganglia, and thalamic structures). In patients, a Chiari II malformation is associated with a neural tube closure defect in almost 100% of cases, usually a lumbosacral myelomeningocele. Characteristic features of a Chiari II malformation are subsequently described, not all features need be or are commonly present (Fig. 1.12). By definition, there will be a small posterior fossa with low insertion of the tentorium. The tentorial incisura may be widened, allowing the cerebellum to extensive superiorly, a “towering cerebellum.” In this instance the folia of the cerebellum will have a vertical orientation. In a small number of cases the cerebellar hemispheres extend more anteriorly than normal, forming on an axial image the appearance of three bumps, the middle being that of the pons. The fourth ventricle is typically elongated (slit-like) and inferiorly displaced. A ballooned fourth ventricle is seen in 10%. Another defining feature is inferior displacement and elongation of the brainstem, tonsils, and vermis. The degree of displacement is often substantial. Cervicomedullary kinking, overlapping of the medulla and cervical cord, may occur. There may be an enlarged foramen magnum and upper cervical canal, accompanied by a smaller C1 ring, with resultant compression of displaced brainstem, tonsils, and vermis at this level. Cervical (and thoracic) syringohydromyelia is common. Obstructive hydrocephalus is usually present, with most patients shunted. Callosal dysgenesis (usually partial agenesis of the corpus callosum) is seen in 75%. Fusion of the colliculi, “tectal beaking,” is seen in the majority. The frontal horns may have a characteristic inferior pointing seen on coronal images. The massa intermedia is typically large. There is often hypoplasia or fenestration of the falx, with interdigitation of cerebral gyri. Stenogyria (multiple small cerebral gyri) is common. A Chiari III malformation is rare, with features of the Chiari II malformation together with a low occipital or upper cervical encephalocele. Fig. 1.11 Chiari I, with evaluation of CSF flow. The cerebellar tonsils are pointed, and extend below the C1 level. Note the normal configuration of the fourth ventricle. Phase contrast images at peak flow velocity in both the cranial and caudal directions are also illustrated. Flow is depicted as white or black, depending on direction, and is seen both anterior to the pons and anterior to the cord, establishing patency at the foramen magnum. An additional concordant finding is visualization of CSF anterior to the brainstem on the sagittal T2-weighted scan at that level. Fig. 1.12 Chiari II. This hindbrain dysgenesis has many characteristic features, not all of which will be present in the same patient. Illustrated are the peglike tonsillar herniation (1), a slitlike fourth ventricle (2), fusion of the colliculi with a beaklike appearance (3), partial agenesis of the corpus callosum (4], a large massa intermedia (5), low insertion of the tentorium (6), a towering appearance to the cerebellum with vertically oriented folia extending through a large tentorial incisura (7), partial agenesis of the falx with interdigitation of gyri (8), and anterior displacement of the cerebellar hemispheres relative to the pons (9). In a Dandy-Walker malformation, there are three primary features. The posterior fossa is large, with the confluence of the sinuses/torcula high in position. Additionally, there is a large posterior fossa cyst that communicates with the fourth ventricle anteriorly. The third defining feature is vermian and cerebellar hemisphere hypoplasia, which can be present to a varying degree (Fig. 1.13). On sagittal images, the residual vermis may be rotated superiorly (counterclockwise). The occipital bone may be scalloped and thinned. Imaging in the sagittal plane is essential for definition of the structural abnormalities. There is a spectrum of severity of findings, and thus the additional use, in the past, of the terms Dandy-Walker continuum and Dandy-Walker variant. The term Dandy-Walker spectrum has also been suggested more recently, specifically including mega cisterna magna as the entity with the mildest findings. Fig. 1.13 Dandy-Walker malformation. There is dilatation of the fourth ventricle (which communicates with a posterior fossa cyst), hypoplasia of the inferior vermis, rotation of the vermis superiorly, and elevation of the torcula. Hemimegalencephaly is defined by hamartomatous over-growth of a hemisphere. The lateral ventricle on the abnormal side is often large. Abnormalities of the brain on the side of involvement are common and include a thickened cortex and abnormal white matter signal intensity. In heterotopic gray matter, there are displaced masses of gray matter, found anywhere from the embryologic site of development (periventricular) to the final destination after cell migration (cortical). The most common presentation is that of small focal regions of gray matter adjacent to the lateral ventricles (Fig. 1.14). Important for diagnosis is that these small focal lesions are isointense to gray matter on all MR pulse sequences. The primary differential diagnostic consideration is tuberous sclerosis, specifically the subependymal nodules therein. The latter may demonstrate calcification, with the cortical involvement in tuberous sclerosis an additional differentiating feature. Less common forms of heterotopic gray matter include large nodular lesions and band heterotopia. Fig. 1.14 Heterotopic gray matter. On CT, a small round lesion projects into the atrium of the lateral ventricle, with a suggestion that the attenuation is that of gray matter. On MR, this small heterotopic focus is isointense to and easily identified as gray matter. Isointensity to gray matter was confirmed on all pulse sequences (not shown). Lissencephaly type 1 or classic lissencephaly (previously known as the pachygyria-agyria complex) is a disorder of cortical formation, with arrested neuronal migration. On imaging, there is a thickened cortex, a smooth brain surface, a small number of shallow sulci, and decreased peripheral arborization of white matter (Fig. 1.15). Histologically there is a four-layered cortex (as opposed to the normal six layers). On a T2-weighted scan, a hyperintense cell sparse zone may be recognizable, separating a thin gray matter cortical ribbon from a thicker underlying gray matter layer containing disorganized neurons. Incomplete lissencephaly (Fig. 1.15), with focal areas of pachygyria (and otherwise normal brain), is much more common than complete lissencephaly. Lissencephaly type 2, also known as cobblestone lissencephaly, is a distinct, separate entity from lissencephaly type 1. In type 2, there is a nodular or “pebbly” appearance to the brain surface, with broadened gyri and loss of sulci (thus the term lissencephaly). There are commonly associated ocular anomalies, with this entity usually occurring as part of a congenital muscular dystrophy. The brain is small and the cerebral white matter reduced in volume. In polymicrogyria, an abnormality involving late neuronal migration, there are multiple small gyri along the brain surface (Fig. 1.15). Due to their very small size, these may not be visualized on MR. The resultant imaging appearance is that of a focal region of brain with an irregular cortical surface, a thick layer of gray matter (cortex), and subtle irregularity at the gray–white matter interface. There will be a decreased number of gyri, with the visible gyri being broad, thick, and relatively smooth. The involvement can be unilateral, bilateral, symmetric, or asymmetric. A perisylvian location is common. Anomalous venous drainage, often a large draining vein located in a deep sulcus, is common in regions of dysplastic cortex. On histology, in polymicrogyria, there is a derangement of the normal six-layered cortex. Schizencephaly is characterized by the presence of a gray matter lined cleft, which extends from the cortex to the ventricular system. Schizencephaly can be either unilateral or bilateral. There is a spectrum of appearance, in terms of separation of the gray matter lined walls, from closed to open lip (Fig. 1.16). A dimple in the wall of the ventricle can be an important clue for recognition of the closed lip form (Fig. 1.17). The septum pellucidum is commonly absent. MR is markedly superior to CT for detection of the gray matter lined cleft and associated abnormalities. Identification of the gray matter lining is important to differentiate schizencephaly from porencephaly. In the latter, there is an abnormal CSF space, due to destruction of brain, which can communicate with the ventricle but is not lined by gray matter. Porencephaly is caused by a vascular accident during the third trimester of fetal development, and as such often abuts the ventricle, with an intervening intact ependyma. The term porencephaly has also been used more generally to include any non-neoplastic cavity within the brain, not specifically in utero in etiology, including vascular insult, trauma, infection, and surgery. Fig. 1.15 Other congenital brain malformations. Six patients are illustrated. In the first there is near complete agenesis of the corpus callosum, with radially oriented gyri adjacent to the lateral ventricles. Many posterior fossa malformations are associated with callosal agenesis, and in this patient a large retrocerebellar cyst is also present. In the second patient, the lateral ventricles demonstrate a parallel orientation, a sign in the axial plane of callosal agenesis. Semilobar holoprosencephaly is illustrated in the third patient, with the interhemispheric fissure absent anteriorly and fused thalami. Rudimentary gyri and a smooth brain surface are seen in the fourth patient, with agyria, who also demonstrates the characteristic “cell-sparse” layer (small black arrows) underlying the cortex. The fifth patient demonstrates pachygyria, with a section (white arrows) of smooth thickened cortical gray matter with shallow sulci. The sixth patient has a focal area in the frontal lobe of polymicrogyria (small black arrows), with many very small gyri producing a cobblestoning appearance. The corpus callosum (CC) is the largest interhemispheric commissure in the brain. The CC is divided into the genu (the “knee”) anteriorly, the body, and the splenium posteriorly. There is an additional small named segment, the rostrum, which is a continuation of the genu, and projects posteriorly and inferiorly (coming from the Latin and meaning “beak,” as with a bird). Other links between the two cerebral hemispheres include the anterior and posterior commissures. The line connecting the two commissures, the AC–PC line, today defines the standard axial plane for MR imaging acquisitions. Fig. 1.17 Schizencephaly, “closed lip.” On the right, a gray matter lined cleft (white arrow) is noted extending from the surface of the brain to the ventricle (continuity with the lateral ventricle was demonstrated on the adjacent section, not shown). On the left, a ventricular dimple (black arrow)—the ependymal margin of the contralateral cleft—is demonstrated, together with part of a gray matter lined cleft, in this patient with bilateral “closed lip” schizencephaly. The corpus callosum develops in the fetus between the 8th and 20th weeks, in an anterior to posterior fashion. The genu forms first, then the body, then the splenium, with the exception that the rostrum forms last. Total agenesis of the corpus callosum is due to an early insult, with partial agenesis due to a later insult during gestation. With total agenesis, axons that normally cross the midline instead run along the medial borders of the lateral ventricles, parallel to the interhemispheric fissure, forming the bundles of Probst (Fig. 1.15). The lateral ventricles will be more widely separated than normal, and their orientation parallel. On a coronal image, there will be a crescent shape to the lateral ventricles, in particular the front horns, with the bundles of Probst lying medially. This appearance has been referred to as “devil’s horns.” There may be dilatation of the trigones of the lateral ventricles and the occipital horns, referred to as colpocephaly. Along the midline, as seen in the sagittal plane, there will be a radial orientation to the gyri adjacent to the body of the lateral ventricles. The term callosal dysgenesis includes both partial and complete absence of the corpus callosum. In most instances, callosal dysgenesis is associated with another anomaly, with the most common being the Chiari II malformation. An excellent survey of the corpus callosum is obtained with sagittal imaging on MR imaging. Holoprosencephaly is a congenital malformation of the brain, characterized by failure of cleavage and differentiation involving the forebrain (prosencephalon). The prosencephalon separates early in development into the diencephalon (which includes the thalamus and hypothalamus) and the telencephalon (which includes the cerebral hemispheres and basal ganglia). Holoprosencephaly is somewhat artificially divided into three subcategories, which are subsequently discussed in order of decreasing severity. The septum pellucidum is absent in all. In alobar holoprosencephaly, the thalami are fused, the third ventricle is absent, the falx is absent (there is no interhemispheric fissure), and there is a single crescent-shaped ventricle connected to a large dorsal cyst. Alobar holoprosencephaly is rarely imaged. Infants are typically stillborn, or of short life span. In semilobar holoprosencephaly, the interhemispheric fissure and falx are present posteriorly (Fig. 1.15). There is partial separation of the thalami by a small third ventricle, with rudimentary temporal horns. The splenium of the corpus callosum is present. In lobar holoprosencephaly, the falx and interhemispheric fissure extend into the frontal region. The anterior falx is dysplastic. The frontal horns of the lateral ventricles have an abnormal configuration, and the frontal lobes may be hypoplastic. Inspection of both axial and coronal MR images is important, with the latter in particular useful to demonstrate frontal lobe abnormalities. Septo-optic dysplasia is defined by the presence of an abnormality involving the septum pellucidum and hypoplasia of the optic nerves (Fig. 1.18). There may be mild dysplasia to complete absence of the septum pellucidum. The optic chiasm and nerves will be small, together with a thin pituitary infundibulum. The degree of pituitary dysfunction is variable, with growth hormone deficiency most common. In neurofibromatosis type 1 (NF1) focal areas of signal abnormality (FASI), with abnormal high signal intensity on T2-weighted scans, are seen in the brain in characteristic locations in the majority of preteen children. The most common location for this finding is the globus pallidus (Fig. 1.19). Here the lesions are usually bilateral, but often asymmetric in size. The lesions of the globus pallidus may also be slightly hyperintense on T1-weighted scans. Similar lesions can also be seen, but are less common, in the thalamus, brainstem, and cerebellar white matter. With age, these abnormalities fade, and eventually may no longer be seen on MR. The other common abnormalities seen in NF1 include gliomas involving either the optic nerve or optic chiasm, and sphenoid wing dysplasia in association with a plexiform tumor. Optic chiasm lesions usually have bilateral contiguous optic tract involvement and may demonstrate optic nerve involvement. Cutaneous lesions in NF1 include cafe au lait spots, axillary freckles, hamartomas (Lisch nodules) of the iris, and multiple subcutaneous nodules (peripheral nerve tumors). NF2 is a much less common disease, with the presence on MR of bilateral vestibular schwannomas being pathognomonic (Fig. 1.20). Meningiomas, ependymomas, and other intracranial nerve tumors can also be seen. Fig. 1.18 Septo-optic dysplasia. This entity is characterized, in its most classic presentation, by absence of the septum pellucidum, which is well seen on axial scans, together with a small optic chiasm (white arrow), optic nerves, and pituitary—all well illustrated in the sagittal plane. Tuberous sclerosis has a characteristic imaging presentation on MR, with both subependymal and parenchymal lesions. In a single patient, one or the other of these two abnormalities may dominate. Small subependymal nodules are common, with some projecting into the ventricular system. These are readily recognized on both T1- and T2-weighted scans, and may demonstrate calcification on CT. Characteristically, there will also be multiple parenchymal lesions, which are best visualized on T2-weighted scans (specifically FLAIR) (Fig. 1.21). These parenchymal hamartomas (tubers) involve both gray and white matter, with their epicenter in the subcortical white matter. These are high signal intensity on T2-weighted scans. The term “gyral core” is used for an abnormality confined to the subcortical white matter core of an expanded gyrus. The term “sulcal island” is used to describe involvement of two adjacent gyri, with sparing of the normal intervening cortex lining the sulcus. Mild ventricular enlargement can be present. CT may demonstrate peripheral calcifications and parenchymal hypodensity associated with parenchymal hamartomas. Brain tumors are seen in a small percent of cases. The most common of these is a subependymal giant cell astrocytoma (WHO grade I), characteristically occurring along the wall of the lateral ventricle with obstructive hydrocephalus due to blockage of the foramen of Monro (Fig. 1.22). Cutaneous lesions seen in tuberous sclerosis include leaf-shaped hypopigmented spots (on the trunk and limbs), adenoma sebaceum (small red papules scattered over the face in a butterfly pattern), and rough thickened yellow skin over the lumbosacral region posteriorly (shagreen patch). Fig. 1.19 Neurofibromatosis type 1 (NF1). Slightly asymmetric round foci of abnormal signal intensity (FASI) are seen on FLAIR in a very characteristic location, the anterior globus pallidus (white arrows). There is no abnormal contrast enhancement. Although FASIs may also occur in other locations, this appearance is pathognomonic for NF1. The Sturge-Weber syndrome is also known as encephalotrigeminal angiomatosis. On CT, focal cortical calcification is seen together with associated atrophy late in the disease process. MR demonstrates focal leptomeningeal enhancement, which markedly improves depiction of the disease and its extent of involvement (Fig. 1.23). Late in the disease process gliosis is seen as abnormal high SI on T2-weighted scans in the adjacent white matter. The cutaneous lesion characteristic for Sturge-Weber is a “port wine stain”; however, this lesion need not be present. Fig. 1.20 Neurofibromatosis type 2 (NF2). Bilateral internal auditory canal enhancing lesions are noted (white arrows), each consistent with a vestibular schwannoma. The lesion on the patient’s left is purely intracanalicular. The lesion on the right expands the internal auditory canal and has both intra- and extra-canalicular components. An additional, moderate in size, enhancing extra-axial lesion (black arrow) is noted, invading the left transverse sinus, consistent with a meningioma. Bilateral vestibular schwannomas are pathognomonic for NF2, with meningiomas often also seen, as in this patient. Cerebellar hemangioblastomas can occur in adults in von Hippel-Lindau disease. Lesions characteristic of this autosomal dominant disease include CNS hemangioblastomas, clear cell renal carcinomas, pheochromocytomas, and cysts of the liver and pancreas. Lipomas are rare congenital malformations with a very distinctive appearance and location, regardless of whether imaged by CT or MRI. Lipomas have low attenuation on CT (− 50 to − 100 HU), and there may be associated calcification. Small lipomas can go unrecognized on CT due to the relative poor detectability of dark objects (as opposed to bright objects) on a uniform background. On MR, lipomas will be isointense to fat on all pulse sequences. Their distinctive appearance, and high detectability, on MR is due to the high signal intensity on T1-weighted scans. Depending upon the bandwidth utilized for the pulse sequence, a chemical shift artifact may also be seen (in the readout [frequency encoding] direction). Scans with and without fat saturation can be used for definitive identification of fat. Lipomas are typically asymptomatic. More than half are associated with other brain malformations. The most common location (about half of all lipomas) is interhemispheric, lying superiorly along the corpus callosum (Fig. 1.24), and in this instance often associated with partial agenesis of the corpus callosum. Other characteristic but less common locations include the suprasellar cistern, the quadrigeminal plate cistern (Fig. 1.25), and the cerebellopontine angle. Intracranial vessels and nerves may course through a lipoma. Fig. 1.21 Tuberous sclerosis. The two pathognomic findings in this disease entity are illustrated, with the respective modalities on which they are best visualized. On FLAIR, high signal intensity within the white matter of two gyri (black arrows) with overlying near normal gray matter is seen, consistent with cortical tubers. In a different patient, calcified subependymal nodules are well identified on CT. Fig. 1.23 Sturge-Weber syndrome. Subtle cortical/subcortical low signal intensity on both scans (part 1) reflects dense dystrophic calcification. There is mild associated gliosis, seen as subtle high signal intensity on the proton density–weighted image. Serpentine enhancement is seen post-contrast, reflecting the pial angioma, overlying the gyri and extending within the sulci in the right occipital lobe in this patient. Cortical calcification is seen on CT (part 2), with focal atrophy on MR, in a different patient, classic features for chronic disease in this phakomatosis. A cephalocele is a protrusion of cranial contents through a congenital defect. There are two main types, a meningocele (Fig. 1.26), which contains meninges and CSF, and a meningoencephalocele (Fig. 1.27), which also contains brain. These most commonly occur along the midline. More than 50% are occipital, the most common location, and 15% fronto-ethmoidal. A related entity to the latter, from an embryogenesis perspective, is a nasal dermal sinus. CT defines the bony defect, MR the associated soft tissues and specifically whether brain tissue is present. Craniosynostosis is premature fusion of a skull suture. The cranial sutures normally begin to fuse at age 3 and are completely fused by age 6. Distortion of the shape of the calvarium is predictable based upon the suture(s) involved. Scaphocephaly (dolichocephaly) is due to synostosis of the sagittal suture, resulting in an increased AP dimension (Fig. 1.28). Brachycephaly is used to describe an increase in transverse dimension of the skull, which can be due to synostosis of the coronal or lambdoid sutures bilaterally. Unilateral coronal or lambdoid synostosis is referred to by the term plagiocephaly, with the result being asymmetrical flattening of one side of the skull. Craniosynostosis is best evaluated by high-resolution, low radiation dose, CT with 3D reconstructions to assess the status of the sutures. This section discusses a diverse group of disorders due to inborn errors of metabolism. Imaging is often suggestive of the general diagnosis, but rarely specific for the individual disease. White matter is usually involved, although this may be secondarily. In the chronic phase for each, these diseases share a similar imaging appearance, with atrophy and usually generalized white matter abnormality. The specific genetic defect for each disease, and often its many variants, is usually today well known. These have not been discussed in detail below, as they contribute little to image interpretation. The most common form of this disease presents in the second year of life. The imaging appearance is nonspecific, with symmetric abnormal high signal intensity in the cerebral white matter on T2-weighted scans. This progresses temporally, together with generalized cerebral atrophy. Clinical symptoms begin between age 3 and 6 months. Early features on imaging can be helpful for diagnosis, with involvement of the thalamus, caudate nucleus, and dentate nucleus, in addition to the more nonspecific involvement of the white matter of the corona radiata. In the nuclei, high density is seen on CT, with this finding preceding the generalized low-density in white matter. The thalami may be high signal intensity on T1-weighted scans early in the disease process, another useful finding for diagnosis if present. This leukodystrophy is often associated with enlargement of optic and cranial nerves. Fig. 1.25 Lipoma of the quadrigeminal plate. A common location for small incidental lipomas, such as that illustrated (arrows), is the quadrigeminal plate. In this instance, the lipoma is adjacent to the left inferior colliculus. Chemical shift artifact, seen as a small black line just superior to the lesion on the sagittal image, identifies the lesion as fat. Fig. 1.26 Cephalocele, parietal. A small midline lesion, with a limited skull defect, is noted. In this instance, the lesion contains meninges and CSF without obvious brain tissue (a meningocele). Note the persistent falcine sinus (arrow), a common associated feature of atretic parietal cephaloceles. Of the many types of adrenoleukodystrophy, the childhood cerebral form is the most relevant and most common. This is a disease of young boys, with presentation between 5 and 12 years of age. Early clinical features include decreased visual acuity, gait disturbances, and mild intellectual impairment, with rapid disease progression. The most common imaging pattern is that of posterior white matter involvement, including specifically the periatrial (parieto-occipital) white matter, the fornix and the splenium of the corpus callosum. The pattern of spread is from posterior to anterior, as opposed to other leukodystrophies that extend from anterior to posterior (i.e., Alexander disease) (Fig. 1.29). Fig. 1.27 Cephalocele, nasoethmoidal. In part 1, coronal and sagittal reformatted CT images are presented, reconstructed with soft tissue and bone algorithms. A well-delineated, somewhat heterogeneous, soft tissue mass—with a suggestion of two components (the more superior portion being isodense to brain), extends through a bony defect into the nasal cavity (ethmoid region). The cribriform plate is deficient. In part 2, two different sagittal midline T2-weighted scans are presented: the first, a 2-mm FSE scan, and the second, a 1-mm CISS (FIESTA-C or bFFE) scan. Both display a linear structure (arrow), isointense to brain, that could be traced on adjacent images and is one of the two olfactory tracts. The tract enters the more superior of the two components of this cephalocele, with the more inferior containing only CSF. This anomaly is thus by definition a meningoencephalocele, containing meninges, CSF, and brain tissue. As to be anticipated, these areas display low density on CT and increased signal intensity on T2-weighted MR scans. The anterior disease margin (the leading margin of demyelination) may display abnormal contrast enhancement, due to its inflammatory nature. In chronic disease, there is atrophy of the splenium. In 15% of patients, the pattern is predominantly frontal in location, with again abnormal contrast enhancement at the peripheral disease margin. In rare instances, the disease is predominantly unilateral. Imaging findings are nonspecific, with abnormal white matter hyperintensity on T2-weighted MR sequences due to delayed/defective myelination. The classic form of this disease presents in the first few days of life. Profound edema is seen in regions of the brain that are normally myelinated at birth. There may be in addition generalized edema of the cerebral hemispheres. The classic form of this disease, a hypomyelinating disorder, is X-linked recessive and presents within the first few months of life. In the most common form of the disease, myelination does not appear to progress further than that normally present at birth. The amount of myelination and white matter slowly decrease with time. This autosomal dominant disease is characterized by degeneration and volume loss involving the corpus striatum (the caudate nucleus and putamen). The most common imaging finding is volume loss involving the head of the caudate nucleus, symmetrically, best demonstrated on thin section heavily T1- or T2-weighted (for good gray–white matter delineation) coronal images (Fig. 1.30). In longstanding disease there is generalized cerebral atrophy. Huntington disease presents clinically in the fourth and later decades, with choreoathetosis and progressive dementia. MR findings are not seen early in the disease process. Fig. 1.29 Adrenoleukodystrophy. Images are presented from a young boy, with males almost exclusively involved in this X-linked disorder, the most common enzyme deficiency disease to present in childhood. The classic pattern of involvement is posterior-predominant, with involvement of the splenium of the corpus callosum, adjacent white matter, and fornix. These findings are reflected in the presented case with abnormal high signal intensity on T2- and low signal intensity on T1-weighted images. This autosomal recessive disease presents in the first few weeks of life due to marked hypotonia, with early development of macrocephaly and seizures. In infantile onset patients, imaging studies demonstrate a nonspecific, symmetric diffuse abnormality of the cerebral white matter. The subcortical white matter is involved early in the disease process, a possible differentiating finding. There is typically involvement of the globus pallidus, with the putamen spared. As with this general category of disease, end-stage findings include atrophy of both white matter and the cerebral cortex, and ventriculomegaly. This disease was originally described as a disorder of infants, with macrocephaly. The infantile form has a rapid lethal course. Together with Canavan disease, it is one of the two leukodystrophies with macrocephaly. Subsequent to the discovery of the specific mutation involved, this disease was shown to have a large spectrum of phenotypes, with juvenile and adolescent forms, and survival into adulthood by some patients. In the infantile form, frontal white matter involvement predominates, with progressive posterior extension. In this category of disease, there is a deficiency of lysosomal enzymes necessary for the degradation of mucopolysaccharides. These diseases are thus multisystemic. Excretion in the urine of incompletely degraded mucopolysaccharides is characteristic. The imaging presentation is one of dilated perivascular spaces (an identifying feature), atrophy with varying degrees of hydrocephalus, together with white matter changes that are initially more focal in nature (Fig. 1.31). These lesions progress with time to resemble a nonspecific metabolic disorder, but if treated early by bone marrow transplantation may regress. Stenosis at the craniovertebral junction is a known additional associated finding. The mucopolysaccharidoses include Hurler (most common), Hunter (next most common), Sanfilippo, and Morquio diseases. Fig. 1.31 Hunter syndrome. Late in the disease process, most of the leukodystrophies cannot be differentiated, and feature generalized cerebral atrophy (reflected by ventricular enlargement in this pediatric patient) together with patchy to diffuse abnormal high signal intensity white matter on T2-weighted scans. However, on the T1-weighted scan illustrated, there is a finding that is characteristic for the mucopolysaccharidoses, specifically Hunter and Hurler diseases, and that is the numerous strikingly enlarged dilated perivascular spaces (arrows). This entity refers to a group of disorders that present with stroke-like symptoms. Presentation is most common in the second decade of life. Patients have in common deletions of mitochondrial DNA. The parietal and occipital cortex and subcortical white matter are most frequently involved, although any area of the brain may be affected. The imaging presentation is one of vasogenic edema, in involved regions, with subsequent resolution and development later of other regions of involvement. Lesions do not follow specific arterial distributions, a differentiating feature from thrombotic or embolic infarction. This term is now known to refer to a symptom complex, with several different genetic causes. The unifying theme appears to be defective terminal oxidative metabolism. Clinical presentation is in the first year of life. The patterns of injury vary with the mutation. Fig. 1.32 Glutaric acidemia type 1. Widened CSF spaces, which include the sylvian fissures and the potential space anterior to the temporal lobes (with these two areas communicating) are characteristic for glutaric acidemia type 1, as shown. Additional typical findings, more difficult to recognize due to the age of this patient (6 months), include increased signal intensity on T2-weighted images in the periventricular white matter as well as the globus pallidus and putamen (arrow) bilaterally. Early recognition of this entity is important, as it is readily treated and otherwise leads to permanent sequelae. A prominent clinical feature of this autosomal recessive disease is presentation with an acute encephalopathy, typically by 18 months of age. Unlike the majority of the genetic metabolic disorders, imaging findings are more specific for this diagnosis, with identification and clinical follow-up critical for treatment. Open Sylvian fissures (due to hypoplasia of the frontal and temporal opercula) together with bilateral basal ganglia (most notably the putamen) involvement (T2 hyperintensity) and macrocephaly are characteristic (Fig. 1.32). A suspicion on imaging of this diagnosis should prompt laboratory evaluation, with dietary intervention known to prevent the devastating neurologic consequences of this disease. These are rare lysosomal storage diseases (Fig. 1.33) caused by deficiency in activity of specific lysosomal enzymes, with GM2 gangliosidosis more widely known due to Tay-Sachs disease (one of the GM2 gangliosidoses, which also include Sandhoff disease). The thalami and white matter are involved early, with cerebral and cerebellar atrophy a late finding. Fig. 1.33 GM1 gangliosidosis. Knowledge of the patient’s age and presentation is critical for image evaluation in a patient suspected to have an inherited metabolic disorder. The image presented is from an 11-month-old patient with cessation of normal development at 3 months of age. Myelination would be appropriate in the axial image presented for a newborn, with high signal intensity in the posterior limb of the internal capsule, but is markedly delayed for a child near 1 year of age. At that time in development, myelination on T1-weighted images should appear near complete, with high signal intensity seen throughout the cerebral and cerebellar white matter. This entity, also known by the term posterior reversible encephalopathy syndrome (PRES), is caused by acute severe hypertension. There is a predilection for involvement of the parietooccipital regions (the posterior circulation), with bilateral, symmetric abnormal high signal intensity (vasogenic edema) on fluid attenuated inversion recovery (FLAIR) involving the cortex and subcortical white matter in a nonvascular distribution (Fig. 1.34). In prominent disease, the involvement of the cerebral hemispheres may be more extensive. Diffusion is usually not restricted. There may be accompanying involvement of the basal ganglia. This entity is caused by thiamine deficiency, and is seen in severe malnutrition, in particular with alcohol abuse. On FLAIR, in extensive disease, abnormal bilateral hyperintensity can be noted in the mamillary bodies, around the third ventricle, and in the medial thalami, tectal plate, and periaqueductal area (Fig. 1.35). Involvement of the mamillary bodies is a distinctive feature. The mamillary bodies can also have abnormal contrast enhancement, as can occur in the other involved areas. Fig. 1.34 Posterior reversible encephalopathy syndrome (PRES). In this entity, also known as acute hypertensive encephalopathy, symmetric abnormal high signal intensity is seen on FLAIR scans, consistent with vasogenic edema, posteriorly in the parietal-occipital regions involving cortical gray and subcortical white matter. On DWI, these areas are most commonly normal, but may be hyperintense (as illustrated), however, with the ADC map normal (no restricted diffusion). Fig. 1.35 Wernicke encephalopathy. Involvement of the mamillary bodies, seen on FLAIR with abnormal hyperintensity (image not shown) and post-contrast with abnormal enhancement (illustrated, arrows), is the most characteristic finding in this disease from an imaging perspective. This entity can occur with either acute or chronic liver disease, and is characterized on MR imaging by high signal intensity bilaterally within the globus pallidus on T1-weighted images (Fig. 1.36). The signal intensity characteristics are thought to reflect manganese deposition. Similar findings can be seen with hyperalimentation. Inhalation of carbon monoxide (CO) results in impaired oxygen transport, with CO having 200 times the affinity for hemoglobin of oxygen. The hallmark of CO poisoning is symmetric injury to the globus pallidus. Initially vasogenic edema will be present, with mild enlargement of the nuclei. With time, this is replaced by gliosis and cystic encephalomalacia, with the chronic appearance being one of symmetric atrophy of the nuclei (Fig. 1.37). Pantothenate kinase-associated neurodegeneration (PKAN) can mimic the appearance of CO poisoning on CT and MR, with the term “eye of the tiger” used for the imaging presentation on T2-weighted scans and FLAIR. This progressive neurodegenerative disorder involves a mutation in the pantothenate kinase 2 gene, and today is classified within the category of neurodegeneration with brain iron accumulation (NBIA), of which PKAN is the most common type. Fig. 1.37 Carbon monoxide poisoning (chronic). The globus pallidus is small (atrophic) bilaterally, with symmetric lesions therein demonstrating peripheral gliosis and central cavitation (fluid). On FLAIR, the gliosis is seen as abnormal high signal intensity, with central lower signal intensity due to fluid. On the heavily T2-weighted fast spin echo scan, the very high signal intensity fluid centrally within the lesions dominates the image appearance, with the gliosis less evident. Fig. 1.38 Osmotic demyelination. There is abnormal high signal intensity centrally within the pons on the axial T2-weighted and FLAIR images presented, with corresponding abnormal low signal intensity on the T1-weighted scan. Note that the lateral and more posterior portions of the pons are spared, together with the more anterior (ventral) pons and the corticospinal tracts. There is also restricted diffusion (which can be seen acutely), with abnormal high signal intensity on DWI, in this comatose patient 1 week following rapid correction of hyponatremia. This disease, previously referred to by the term central pontine myelinolysis, occurs due to too rapid correction of severe chronic hyponatremia (often in patients with alcoholism or malnutrition). In its classic presentation, there is abnormal symmetric involvement of the central pons, sparing the periphery, with high signal intensity on T2-weighted scans (Fig. 1.38) (which may lag behind clinical symptoms by 1 to 2 weeks) and restricted diffusion (seen early in the disease process). Extrapontine myelinolysis is most commonly seen in conjunction with central pontine myelinolysis (the term osmotic demyelination encompasses both entities), with symmetric involvement of the basal ganglia and cerebral white matter, and less commonly other areas. The etiology of mesial temporal sclerosis is unknown, with this entity likely the end result of several different possible insults. On pathology, there is atrophy of the hippocampus and adjacent structures, with disease bilateral in up to 20%. Mesial temporal sclerosis is a common cause of complex partial seizures. Thin section, high-resolution, coronal MR scans reveal atrophy of the hippocampus and ipsilateral fornix, with resultant mild enlargement of the adjacent temporal horn (Fig. 1.39). Thin section coronal T2-weighted or FLAIR scans may also demonstrate hyperintensity of the involved hippocampus, with loss of the normal internal architecture common. Fig. 1.39 Mesial temporal sclerosis. In part 1, on the coronal thin section (2.4 mm) FLAIR image, there is gross atrophy of the right hippocampus with enlargement of the adjacent temporal horn of the lateral ventricle (asterisk). Note the preservation of architecture and distinct layers of gray and white matter in the normal left hippocampus (white arrow). The diagnosis was confirmed in this instance by surgical pathology. In part 2, thin section (1 mm) contiguous coronal T1-weighted scans confirm the atrophy of the right hippocampus and widening of the adjacent anterior temporal horn (asterisk). These sections also demonstrate that the right hippocampus is small, when compared to the left, throughout its extent from anterior to posterior (black arrows). Hemorrhage has a specific but varied appearance on MR, dependent on time frame (Fig. 1.40). The appearance is much more straightforward on CT. In normotensive young adults, vascular malformations are the most common cause of spontaneous hemorrhage. In adults, parenchymal hemorrhage is commonly due to hypertension (Fig. 1.41), whereas subarachnoid hemorrhage is commonly due to rupture of an intracranial aneurysm. Typical locations for hypertensive hemorrhage include, in order of decreasing frequency, the basal ganglia (in particular the putamen), thalamus, pons (Fig. 1.42), and cerebellar hemisphere. The descriptions of the appearance of hemorrhage in the literature are predominantly for parenchymal bleeds. To some extent, the appearance of subarachnoid hemorrhage is similar. Fig. 1.40 Temporal evolution of parenchymal hemorrhage. On initial presentation, this posterior temporal hematoma (white arrow) demonstrates low signal intensity on the T2-weighted scan, indicative of deoxyhemoglobin. Also present is surrounding vasogenic edema, with abnormal high signal intensity. Two weeks later, temporal evolution has occurred to extracellular methemoglobin, with high signal intensity on the T2-weighted scan. Five months following presentation, there has been resorption of most of the fluid, together with resolution of the edema, leaving a low signal intensity hemosiderin cleft (black arrow). Hyperacute hemorrhage on CT is of moderate density, rapidly increasing further in density (attenuation) over the first few hours due to clot formation and retraction. After a few days, in the subacute time frame, a progressive loss in attenuation begins. By 1 to 4 weeks, a hematoma will be isodense to brain, and in the chronic phase may appear hypodense. The subsequent description of hemorrhage on MR is for field strengths of 1.5T and above, covering the vast majority of clinical systems today. Magnetic susceptibility effects, which cause decreased signal intensity depending on the time frame of the hemorrhage, are much less evident at lower field strengths. On MR, hemorrhage follows a regular well-defined temporal progression of changes in signal intensity (Fig. 1.43). Oxyhemoglobin (hyperacute) progresses to deoxyhemoglobin (acute), to intracellular methemoglobin (early subacute), then extracellular methemoglobin (late subacute), and eventually to hemosiderin (chronic). Oxyhemoglobin (hyperacute hemorrhage) has the signal intensity of fluid, high on T2- and low on T1-weighted scans. This imaging appearance is relatively nonspecific. Within hours, however, deoxyhemoglobin (acute hemorrhage) is evident with distinctive low signal intensity on T2-weighted scans. Deoxyhemoglobin does not have a unique appearance on T1-weighted scans, on which it appears isointense to mildly hypointense. Methemoglobin (subacute hemorrhage) has distinctive high signal intensity on T1-weighted scans, and bleeds can be further subdivided temporally into intracellular and extracellular methemoglobin. Initially, in the intracellular phase, blood will be high signal intensity on a T1-weighted scan and low signal intensity on a T2-weighted scan (the latter due to a susceptibility effect). With red blood cell lysis, methemoglobin becomes extra-cellular in location, with distinctive high signal intensity on both T1- and T2-weighted scans (Fig. 1.44). With time, methemoglobin is converted into hemosiderin (and to a lesser degree ferritin), with chronic hemorrhage thus exhibiting pronounced low signal intensity on T2-weighted scans again due to susceptibility effects. The appearance of a chronic parenchymal hemorrhage on MR also depends on whether the central fluid collection is resorbed or not. If resorbed, a hemosiderin cleft will be left. If not resorbed, there will be a central fluid collection with high signal intensity on both T1- and T2-weighted scans, surrounded by a hemosiderin rim. With the passage of years, the fluid collection may change in appearance on T1-weighted scans from high to low signal intensity. It is important to note that the evolution of parenchymal hemorrhage on MR does not always follow the characteristic pattern described. Additional factors can be very important, including dilution, clotting, and hematocrit. One key to the recognition of parenchymal hemorrhage, not discussed in detail, is the presence of edema surrounding the hematoma, which is seen in the hyperacute, acute, and early subacute stages. Fig. 1.41 Hypertensive hemorrhage. A large acute parenchymal hematoma is seen on the initial CT in this patient (left upper image), with its epicenter in the right putamen. There has also been extravasation of blood into the ventricular system, with hemorrhage seen in the frontal horns, third ventricle, and atria of the lateral ventricles. On the follow-up CT 3 weeks later, the hematoma is smaller and is in transition from hyperdense to isodense to brain. On the MR obtained after an additional 3 weeks, the hematoma is high signal intensity on both T2- and T1-weighted scans (consistent with methemoglobin), with a hemosiderin rim seen on the T2-weighted scan and a thin peripheral rim of enhancement (black arrow) post-contrast. Both are characteristic features on MR in a subacute hematoma, with the enhancement blending into the margin of the high signal intensity hemorrhage. Fig. 1.42 Acute pontine hemorrhage. A large acute hemorrhage is seen centrally in the pons on axial unenhanced and sagittal enhanced CT. A small amount of hemorrhage is also seen in the fourth ventricle posteriorly, which is compressed due to mass effect. The pons and cerebellum, considered together, are the third most common site for hypertensive hemorrhage, after the putamen/external capsule (first) and the thalamus (second). This known hypertensive patient presented in hypertensive crisis. Fig. 1.43 Parenchymal hemorrhage on MR, the spectrum of signal intensity appearance. Initially, but for a very short time, a parenchymal hemorrhage contains oxygenated hemoglobin and is seen as a fluid collection, with slight hyperintensity on a T2-weighted scan. A large, basal ganglia, hyperacute hemorrhage (black asterisk) illustrates this appearance. There is a small amount of associated vasogenic edema and marked mass effect upon the right lateral ventricle. In the next patient, FLAIR scans are shown both on presentation and long term follow-up. This large, acute, left external capsule hematoma evolves from a fluid collection containing deoxyhemoglobin (white asterisk, with low signal intensity on T2-weighted scans), to a hemosiderin lined cleft (also low signal intensity). Note the associated vasogenic edema and mass effect in the acute stage. The third patient illustrates a subacute, extracellular methemoglobin hematoma, with high signal intensity on both T2- and T1-weighted scans, in the left parietal region. Note the peripheral hemosiderin rim, with low signal intensity, already present on the T2-weighted scan. The fourth patient illustrates on an unenhanced T1-weighted scan a methemoglobin hematoma in the right occipital lobe. This case emphasizes the importance of looking for the cause of a hemorrhage, as the post-contrast scan reveals the associated enhancing metastasis (black arrows). The appearance of acute subarachnoid hemorrhage on CT is generally well known, being well visualized with abnormal high attenuation. Depending on the amount of blood present, the sensitivity for detection of subarachnoid hemorrhage on CT can decrease rapidly with time following presentation. CT can be negative in patients with subarachnoid hemorrhage due either to a time delay (a few days) between the hemorrhage and imaging or the small quantity of blood present. MR is actually more sensitive for subarachnoid hemorrhage than CT, although the imaging appearance is complex and recognition thus more difficult, particularly for radiologists with less experience. FLAIR is extremely sensitive to changes in the CSF, thus even very small amounts of subarachnoid hemorrhage will be seen as abnormal high signal intensity within the sulci. This appearance is however not specific for subarachnoid hemorrhage, and can be seen in any disease process that leads to a subtle change from normal in the composition of CSF. Meningitis produces this appearance, with administration of 100% O2 in anesthetized ventilated patients another known cause. Regional versus global distribution of changes on FLAIR however aids in differentiation of these processes. Fig. 1.44 Parenchymal hemorrhage, involving the globus pallidus and putamen (the lentiform nucleus). High signal intensity on both T1- and T2-weighted scans is consistent with extracellular methemoglobin, in this late subacute hypertensive hemorrhage. An additional characteristic finding is the faint hemosiderin rim, with low signal intensity on the T2-weighted scan. On T2* (susceptibility) weighted images, acute subarachnoid blood will be seen as low signal intensity, in distinction to normal high signal intensity CSF. In combination with the appearance on FLAIR, this finding is specific for acute subarachnoid hemorrhage (Fig. 1.45). Depending on the time frame and the amount of hemorrhage, subarachnoid hemorrhage can also be seen as high signal intensity on T1-weighted scans, due to the presence of methemoglobin. By 3 days following presentation, high signal intensity is commonly seen on T1-weighted scans, a finding that typically persists for days to weeks. In comparison, the sensitivity of CT to subarachnoid hemorrhage decreases markedly following the first few days, with CT often normal thereafter. Hemorrhage within the ventricular system is similarly well seen in the acute time frame by CT, and both acutely and later on following clinical presentation by MR. Blood clots are common within the ventricular system, with hyperdensity on CT and a variable but characteristic appearance on MR depending on composition. Layering of a small amount of hemorrhage posteriorly in the atria of the lateral ventricles is commonly visualized on MR, a sign that can persist for days to weeks following hemorrhage. In superficial siderosis, there is hemosiderin deposition in macrophages within the membranes lining the CSF spaces. The cause is recurrent subarachnoid hemorrhage, typically due to a hemorrhagic neoplasm, ruptured aneurysm, or vascular malformation that has bled. The surface of the cerebellum is the most common site (Fig. 1.46). Clinical symptoms occur rarely, and only when there is substantial deposition of hemosiderin. Possible symptoms include sensorineural hearing loss, pyramidal tract signs, and cerebellar dysfunction, together with cranial nerve dysfunction (most often cranial nerves II, V, VII, or VIII, with the severity of injury proportional to the cisternal length of the nerve). T2-weighted scans at 1.5T and above demonstrate hypointensity of the involved leptomeninges or ependyma. Any sequence that improves the sensitivity to susceptibility (T2*), such as a gradient echo sequence or susceptibility weighted imaging, will also be more sensitive to the presence of superficial siderosis (as well as the use of 3T as opposed to 1.5T). Fig. 1.45 Acute subarachnoid hemorrhage, MR. On initial inspection, the T2-weighted scan appears normal (other than a small external capsule chronic lacunar infarct). However, in retrospect (particularly in comparison with the GRE scan), the left sylvian fissure does not demonstrate the characteristic high signal intensity of CSF (it is isointense with brain), raising the suspicion of subarachnoid hemorrhage or other pathology. FLAIR confirms this finding, but also demonstrates abnormal high signal intensity in two sulci posteriorly, indicative of either blood or inflammatory changes. The GRE scan confirms that both findings represent subarachnoid hemorrhage (arrows), with abnormal low signal intensity due to T2* changes (seen with both deoxy- and intracellular methemoglobin). Fig. 1.46 Superficial siderosis. There is somewhat subtle abnormal hypointensity surrounding the pons, and outlining the superior cerebellar folia, due to superficial hemosiderin deposition from recurrent hemorrhage. A few definitions are in order to introduce the topic of brain trauma. A cortical contusion is simply a bruise of the brain’s surface. The inferofrontal and anteroinferior temporal portions of these two lobes of the brain are particularly vulnerable (Fig. 1.47). The term “coup” is used to reference an injury that lies directly beneath the area of impact. The term “contrecoup” is used for an injury that occurs remote from the site of impact, along a direct line but opposite to this site, caused by acceleration effects. Imaging of acute head injury is the province of CT. Acute intracranial hemorrhage and bone fractures are well seen and evaluated (Fig. 1.48). Diffuse axonal injury (DAI) and cortical contusion are the two most common findings in the setting of trauma with a closed head injury. Subcortical gray matter injury also occurs, but much less frequently. DAI occurs due to shear-strain forces with rapid deceleration. There are three common locations (given in order from the most to the least common, which also parallels the degree of severity of the injury from least to most): the gray–white matter junction, the corpus callosum (splenium), and the brainstem (Fig. 1.49). CT is often normal in this setting. Injuries at the gray–white matter junction will be seen as multiple small foci of abnormal high signal intensity on FLAIR scans. Most commonly these involve the frontal lobe. If the lesions are hemorrhagic, they will be well seen in the acute time frame on T2*-weighted gradient echo scans, due to the presence of deoxyhemoglobin. Susceptibility weighted imaging offers a further improvement in sensitivity to T2* effects, and in cases with hemorrhage will demonstrate more extensive injury. The second most common injury seen in DAI is a shear injury of the corpus callosum, with the majority involving the splenium. This injury and the subsequently described injury of the brainstem are usually not seen in isolation but with extensive shear injury at the gray–white matter junction. In the brainstem, lesions are seen most often in the pons and the dorsolateral midbrain. Lesions in the brainstem carry a very poor prognosis, often with a fatal outcome. In patients evaluated months to years following severe head trauma, the residual from DAI involving injury at the gray–white matter junction can be visualized, with high signal intensity on FLAIR due to gliosis and low signal intensity on T2*-weighted images due to the presence of hemosiderin. Encephalomalacia, with both gliosis and cystic changes, will be seen in areas of prior contusion. In severe injury, there may be resultant generalized cerebral atrophy.
 Normal Anatomy and Common Variants
Normal Anatomy and Common Variants
Normal Intracranial Anatomy
Normal Arterial Anatomy
Normal Venous Anatomy
Normal Myelination
Variants Involving the Septum Pellucidum
Physiological Calcification
Incidental Cystic Lesions
Dilated Perivascular Spaces
Other Incidental Lesions
 Congenital Malformations
Congenital Malformations
Posterior Fossa Malformations
Cortical Malformations
Callosal Malformations
Holoprosencephaly and Related Disorders
Phakomatoses
Lipomas
Anomalies of the Skull
 Inherited Metabolic Disorders
Inherited Metabolic Disorders
Diseases Affecting White Matter
Metachromatic Leukodystrophy
Krabbe Disease
X-linked Adrenal Leukodystrophy
Phenylketonuria
Maple Syrup Urine Disease
Pelizaeus-Merzbacher Disease
Disease Affecting Gray Matter: Huntington Disease
Diseases Affecting Both White and Gray Matter
Canavan Disease
Alexander Disease
Mucopolysaccharidoses
Mitochondrial Encephalomyopathy with Lactic Acidosis and Stroke-like Episodes (MELAS)
Leigh Syndrome
Glutaric Acidemia Type 1
GM1 and GM2 Gangliosidoses
 Acquired Metabolic, Systemic, and Toxic Disorders
Acquired Metabolic, Systemic, and Toxic Disorders
Acute Hypertensive Encephalopathy
Wernicke Encephalopathy
Hepatic Encephalopathy
Carbon Monoxide Poisoning
Osmotic Demyelination
Mesial Temporal Sclerosis
 Hemorrhage
Hemorrhage
Parenchymal Hemorrhage
Subarachnoid Hemorrhage
Superficial Siderosis
 Trauma
Trauma
Parenchymal Injury
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree