Brain Abscess
79.1.1 Overview
Intraparenchymal abscesses require early diagnosis and intervention to minimize morbidity and mortality in the pediatric population. Because of the wide variety of potential pathogens, microbiological evidence through culture data provides a foundation for diagnosis and treatment. Appropriate therapy of intracranial abscesses requires combined surgical and medical management and collaborative care. This chapter highlights the main diagnostic considerations and treatment options for brain abscesses in the pediatric population.
79.1.2 History
William Macewen recorded the first diagnosis of a pediatric brain abscess in 1876 by postmortem examination.1,2 Clinical suspicion arose because of the patient’s symptomatology, while localization stemmed from neurologic assessment. The surgical management of brain abscesses became more widely used in the 1930s, shortly after Walter Dandy recorded the first attempted aspiration of an intracranial abscess in 1926.2 Subsequently, Pennybacker achieved the first safe and effective surgical resection of a cerebellar abscess in 1948.3
79.1.3 Epidemiology
Children age 4 to 7 years comprise the group of pediatric patients most commonly affected by brain abscesses.1 Of all intraparenchymal abscesses, nearly 25% occur in the pediatric population younger than age 15.4 Despite a low incidence of 4 cases per million, intraparenchymal abscesses require close attention in the pediatric population because they carry high risks for morbidity and mortality.1,5
79.1.4 Pathology
An abscess is a well-circumscribed, encapsulated collection of purulent material containing microorganisms, inflammatory cells, cellular debris, and necrotic material.2,6 Abscess formation within the brain parenchyma proceeds through four stages (see box “▶ Stages of Brain Abscess Formation”). The initial stage of early cerebritis involves minimal central necrosis and surrounding edema, with subtle radiographic features. Imaging characteristics become more noticeable during late cerebritis, with early discontinuous sparse enhancement. During this phase, inflammatory cells congregate circumferentially around a necrotic core. Subsequently, during early capsule formation, fibroblasts create a thin capsule that exhibits contrast enhancement on computed tomography (CT) and magnetic resonance (MR) imaging. The late capsule stage displays greater contrast enhancement along a thicker, vascular, and more fibrotic abscess wall that surrounds a necrotic core.1
Stages of Brain Abscess Formation
Stage I: Early cerebritis
Days 1–3
Small area of central necrosis
Surrounding edema
Head computed tomographic findings variable but typically not prominent; hypodensity or faint contrast enhancement possible
Stage II: Late cerebritis
Days 4–9
Necrotic core
Surrounding inflammatory cells
Early patchy enhancement on imaging
Thicker rim of peripheral enhancement later on imaging
Stage III: Early capsule formation
Days 10–14
Necrotic core
Thin capsule formed by fibroblasts
Distinct rim enhancement on imaging
Stage IV: Late capsule stage
Day 15 and beyond
Necrotic core
Thicker vascular capsule with increased fibrosis
Thicker rim of peripheral enhancement on imaging
Source: Adapted from Frazier JL, Ahn ES, Jallo GI. Management of brain abscesses in children. Neurosurg Focus 2008;24(6):E8.1
79.1.5 Pathophysiology
Various mechanisms and underlying conditions account for the occurrence of intracranial abscesses. Potential etiologies include seeding from penetrating trauma, local spread of a contiguous infection, hematogenous spread of a distant infection, predisposition due to congenital abnormalities, and complications of an intracranial procedure. Patient characteristics and environmental factors influence the specific mechanism of brain abscess formation. For instance, neonates and infants display higher rates of cerebral abscess formation due to hematogenous dissemination and bacterial meningitis.1 In developing nations, the contiguous spread of suppurative otitis media is a predominant cause of intraparenchymal abscesses.1,2,7
Contiguous Spread
The intracranial spread of nearby infections typically occurs through necrotic tissue or focal areas of osteomyelitis.5 For instance, contiguous spread via the labyrinthine system or tegmen tympani may account for temporal or cerebellar abscesses resulting from mastoiditis or otitis media.1 Contiguous spread of infection may also occur through the vasculature. Small, valveless diploic veins traverse the sinus walls and communicate with the dural venous plexuses, offering routes of entry for infections involving the paranasal sinuses.5 Local infections predisposing to intraparenchymal abscesses include otic, dental, sinus, facial, and periorbital infections.
Predisposing Conditions
Several predisposing conditions in children relate directly to the mechanisms of intracranial infection (▶ Table 79.1). Congenital cardiac disease is an underlying abnormality in nearly 30% of children with intraparenchymal abscesses.1 Cardiac anomalies include tetralogy of Fallot, transposition of the great vessels, atrial or ventricular septal defects, and Eisenmenger syndrome. The right-to-left shunt created in cyanotic heart disease allows the passage of paradoxical emboli and intracranial seeding via hematogenous spread.1,4,6 Immunosuppression is another important predisposing factor for brain abscess formation. Incompetence of the immune system occurs in the setting of immunosuppression for organ transplant, chemotherapy for malignancy, or underlying diseases like acquired immunodeficiency syndrome (AIDS). Pulmonary conditions, including cystic fibrosis and bronchiectasis, increase the risk for respiratory infections that may lead to brain abscess formation. Intracranial abscesses may also arise because of congenital abnormalities like dermoid cysts and dermal sinus tracts, which allow the direct invasion of microorganisms.8,9
| Predisposing condition | Mechanism of infection | Typical abscess location | Common microorganisms |
| Penetrating head trauma | Direct inoculation | Any intraparenchymal site | Staphylococcus aureus, viridans streptococci, Streptococcus pneumoniae, Enterobacteriaceae |
| Prolonged bacteremia, endocarditis | Hematogenous spread (remote location) | Arterial distribution, especially middle cerebral artery territory | S. aureus, streptococci |
| Sinus or dental infection | Contiguous spread Local venous spread | Frontal lobes | Gram-positive cocci and gram-negative bacilli; streptococci (e.g., Streptococcus milleri), S. aureus, Enterobacteriaceae |
| Chronic otitis media or mastoiditis | Contiguous spread | Temporal lobes, cerebellum | Gram-negative bacilli, streptococci (aerobic and anaerobic), Enterobacteriaceae, Pseudomonas aeruginosa, S. aureus |
| Congenital cyanotic heart disease | Right-to-left shunting, paradoxical emboli | Arterial distribution, especially middle cerebral artery territory | Gram-positive cocci, viridans streptococci |
| Immunocompromised status | Opportunistic infections | Arterial distribution, any intraparenchymal site | Fungi, yeast: Candida, Aspergillus, Mucorales |
| Immunodeficient status (AIDS) | Opportunistic infections | Any intraparenchymal site | Toxoplasma gondii, Nocardia, Mycobacterium |
| Ventriculoperitoneal shunt | Foreign body, ascending infection | Along proximal catheter trajectory; may include ventriculitis | S. aureus, Staphylococcus epidermidis, gram-negative bacilli, P. aeruginosa |
Trauma and Foreign Bodies
Penetrating trauma is a common cause of pediatric intracranial infection due to retained foreign debris or skull fragments. Often planted deep within the brain, such material cannot be removed safely and serves as a nidus for infection.1 Up to 13% of brain abscesses result from penetrating craniofacial trauma. Conversely, in from 5 to 16% of cases, penetrating head trauma leads to intraparenchymal abscess formation.1 Other intracranial foreign bodies, such as ventricular shunts for hydrocephalus, also predispose to abscess formation. Microorganisms may colonize an intracranial catheter or ascend a distal catheter from the peritoneal space. Despite these plausible mechanisms, intraparenchymal abscesses associated with ventricular shunts occur infrequently.10 Although various mechanisms and predisposing conditions have been described, nearly 30% of intraparenchymal abscesses occur without an identified etiology.1,4
Abscess Location
The specific location of an intraparenchymal abscess may reflect the mechanism of disease spread (▶ Table 79.1). For instance, abscesses in the distribution of the intracranial arterial circulation, especially the middle cerebral artery (MCA), suggest hematogenous dissemination from a remote source.4,6 Local venous drainage of sinus, dental, or facial infections into the cavernous sinus may lead to frontal lobe abscesses via retrograde thrombophlebitis.1 Additionally, frontal abscesses may arise from dental or sinus infections via contiguous spread.4,6 Temporal lobe or cerebellar abscesses may suggest direct invasion from mastoiditis or chronic middle ear infections.4–6 Infratentorial abscesses may result from infection of posterior fossa dermoid cysts and associated dermal sinus tracts.3,8 The locations of intraparenchymal abscesses also correlate with age. Younger patients have higher rates of cerebellar abscesses, whereas older children have a higher incidence of temporal lobe abscesses.6
79.1.6 Microbiology
The specific type of infecting microorganism depends on patient characteristics and mechanism of disease (▶ Table 79.2). Gram-positive cocci, including Streptococcus and Staphylococcus species, are the most common group of microorganisms found in pediatric brain abscesses.1,2,6 Gram-negative rods, including the Enterobacteriaceae family and Haemophilus species, are the next most common group of pathogens implicated. Facultative and obligate anaerobic organisms are the predominant isolates reported in intracranial abscesses historically.5
| Domain | Microbiological descriptors | Genus and species | Common patient characteristics | Additional comments |
| Bacteria | Aerobic and facultatively anaerobic, gram-positive cocci | Streptococcus pneumoniae | Meningitis, head trauma | |
| Viridans streptococci | Cyanotic congenital heart disease, head trauma | |||
| Streptococcus milleri group | Child beyond neonatal period | Subgroup of viridans streptococci, also referred to as Streptococcus anginosus; microaerophilic | ||
| Staphylococcus aureus | Head trauma, sinusitis, ventricular shunt, chronic otitis media | Increasing prevalence of methicillin-resistant S. aureus (MRSA) | ||
| Staphylococcus epidermidis | Ventricular shunt | Create biofilms adherent to catheters | ||
| Facultatively anaerobic, gram-positive bacilli | Listeria monocytogenes | Immunocompromised host, neonate | Sensitive to ampicillin | |
| Aerobic, gram-positive bacilli | Nocardia species | Immunocompromised host, AIDS | Often confused with tuberculosis because of acid-fast staining and filamentous structure | |
| Facultatively anaerobic, gram-negative bacilli; Enterobacteriaceae family | Escherichia coli | Ventriculoperitoneal shunt | Sensitive to third-generation cephalosporins | |
| Klebsiella species | Ventriculoperitoneal shunt | Sensitive to third-generation cephalosporins | ||
| Proteus species | Ventriculoperitoneal shunt, otic infection | Sensitive to third-generation cephalosporins | ||
| Enterobacter species | Ventriculoperitoneal shunt, otic infection | Sensitive to fourth-generation cephalosporins | ||
| Citrobacter species | Neonate, infant; meningitis | Most common microorganisms in neonatal brain abscesses | ||
| Anaerobic, gram-negative bacilli | Bacteroides species | Chronic otitis media, mastoiditis, sinusitis | Most common anaerobic organisms found in temporal lobe abscesses due to otic infection | |
| Aerobic, gram-negative bacilli | Pseudomonas aeruginosa | Ventriculoperitoneal shunt, chronic otitis | Also present in immunodeficient hosts | |
| Aerobic and facultatively anaerobic, gram-negative bacilli | Haemophilus influenzae | Meningitis, chronic sinusitis | Sensitive to third-generation cephalosporins | |
| Aerobic, acid-fast bacilli | Mycobacterium tuberculosis | Immunodeficient host, AIDS | ||
| Fungi | Branching pattern of septate hyphae | Aspergillus | Immunocompromised host | Typically spread from paranasal sinusitis or respiratory infections |
| Dimorphic mycelial and yeast forms | Coccidioides immitis | Immunocompromised host | Geographic predominance in southwestern United States, Central and South America | |
| Budding yeast | Cryptococcus neoformans | Immunodeficient host, AIDS | Sensitive to amphotericin B and flucytosine | |
| Protozoa | Infectious cyst form | Toxoplasma gondii | Immunodeficient host, AIDS | Increasing prevalence in regions where AIDS is endemic |
| Trophozoite and cyst forms | Entamoeba histolytica | Developing nations, immigrants to United States | Sensitive to metronidazole | |
Subpopulations of children exhibit trends with respect to infecting microorganisms. For instance, immunocompromised children show a higher frequency of intraparenchymal abscesses infected with fungi, yeast, Nocardia, Toxoplasma gondii, and Mycobacterium tuberculosis.4,5 The rising prevalence of these infections results from increasing rates of AIDS, chemotherapy, and immunosuppression.2 In the neonatal population, Citrobacter is the most common cause of brain abscesses, especially in the setting of meningitis.1,6 Intraparenchymal abscesses associated with cyanotic congenital heart disease tend to be infected with viridans streptococci and other gram-positive cocci.5 Similarly, intracranial penetrating trauma predisposes to brain abscesses infected with Staphylococcus aureus, viridans streptococci, and Streptococcus pneumoniae.5 Intraparenchymal abscesses associated with ventriculoperitoneal shunts frequently exhibit culture isolates of S. aureus and Staphylococcus epidermidis, enteric gram-negative bacilli, and Pseudomonas aeruginosa.5 Delayed abscesses with Propionibacterium acnes may form along a retained ventricular catheter.11 Multiple organisms may be present in up to 30% of brain abscesses; alternatively, many abscesses lack remarkable culture results.4
79.1.7 Clinical Presentation
Generalized Findings
Children with brain abscesses often present with symptoms related to infection and elevated intracranial pressure (ICP). Although common, the classic triad of fever, headache, and focal neurologic deficit is not present in the majority of patients.4,12 Up to 70% of children with an intaparenchymal abscess present with fever.1,4 Furthermore, most pediatric patients with an intraparenchymal abscess present with at least one symptom or sign of elevated ICP, including headaches, emesis, and papilledema.4 Neonates with elevated ICP may show fullness or bulging of the anterior fontanel and increasing head circumference.6
A patient’s neurologic status may be subtle confusion, lethargy, stupor, or coma depending upon the time of presentation and fulminance of disease.6 Coexistent meningitis may contribute to a declining neurologic status.3 Any acute decrement in the examination findings or Glascow Coma Scale score can signify intraventricular extension of the abscess contents, a highly morbid and potentially fatal occurrence.1,6
Neurologic Deficits
As with most intracranial lesions, the location, size, and surrounding edema of a brain abscess dictate the neurologic findings. Focal neurologic signs or seizures occur in 25 to 50% of pediatric patients with brain abscesses.4 Seizures are a presenting symptom in nearly 27% of children with intraparenchymal abscesses.2 Frontal lobe lesions often lead to personality or behavioral changes, difficulty with cognitive processes, or disinhibition.6 Additionally, contralateral motor weakness may develop from mass effect on the motor cortex. Cerebellar lesions may produce gait or limb ataxias, incoordination, eye movement abnormalities, or symptoms of elevated ICP due to obstructive hydrocephalus. Abscesses within the brainstem can cause cranial nerve deficits (cranial nerves III, VI, and VII) or upper motor tract signs.13
Parietal and temporal lobe abscesses cause deficits depending upon laterality and the structures involved. Nondominant parietal lesions may lead to contralateral hemineglect, dyspraxia, and visual field cuts. Abscesses in the parietal lobe of the dominant hemisphere may cause subtle dysphasias and visual field cuts as well. Visual deficits resulting from parietal lesions range from contralateral inferior quadrantanopsia to homonymous hemianopia depending upon involvement of the optic radiations.6 Temporal lobe abscesses may also cause visual field cuts. Additionally, abscesses within the temporal region of the dominant hemisphere may lead to pronounced aphasia.
79.1.8 Diagnosis
Imaging Findings
CT and MR imaging of the head have significantly improved the early diagnosis of brain abscesses. Ultrasonography of the head can also be used intraoperatively or in infants with open fontanels. With greater speed and ease of acquisition than MR imaging, CT of the head typically portrays a centrally hypodense lesion with a peripheral rim of contrast enhancement.2 Surrounding hypodensity typically indicates vasogenic edema (▶ Fig. 79.1). The findings on CT of the head may lag the clinical presentation; therefore, a lack of noteworthy findings should not exclude the diagnosis of brain abscess initially.4
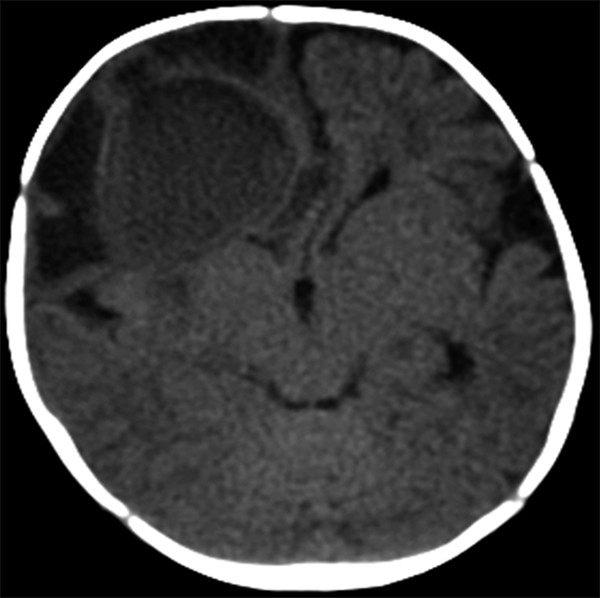
Fig. 79.1 Axial computed tomographic scan of the head (without contrast) showing a right frontal brain abscess with surrounding hypodensity anteriorly and laterally, consistent with vasogenic edema.
Offering greater anatomical detail, MR imaging provides a better characterization of abscess size, location, quantity, and relation to neighboring structures.6 T2-weighted sequences depict a centrally hyperintense lesion with a hypointense rim and surrounding increased signal, consistent with vasogenic edema (▶ Fig. 79.2). Gadolinium administration on T1-weighted imaging portrays a centrally hypointense lesion with a peripheral rim of contrast enhancement (▶ Fig. 79.3). MR imaging also offers enhanced visualization of satellite lesions, cerebritis, and the differentiation between edema and liquefactive necrosis.1 The MR imaging characteristics of an intraparenchymal abscess mirror those of other intracranial lesions in a child; the differential diagnosis broadly includes tumor, tuberculoma, cysticercosis, and vascular lesions.1
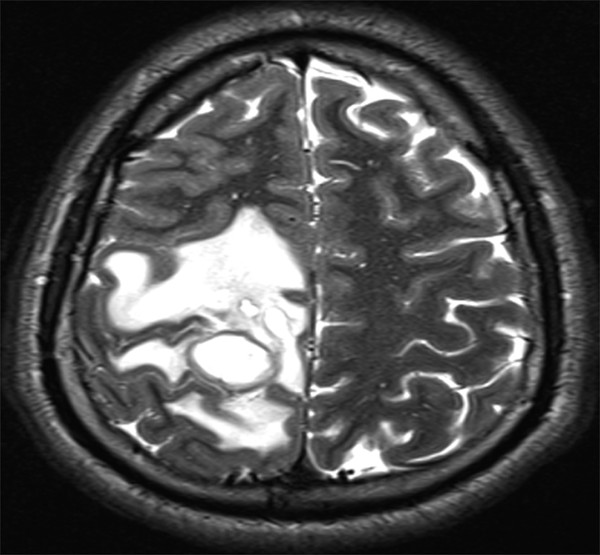
Fig. 79.2 Axial T2-weighted magnetic resonance imaging sequence depicting a right parietal intraparenchymal abscess with surrounding hyperintense vasogenic edema.
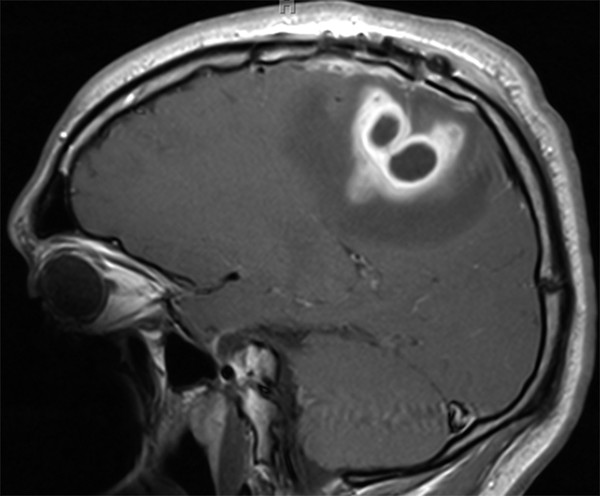
Fig. 79.3 Sagittal T1-weighted magnetic resonance imaging sequence with gadolinium administration showing peripheral contrast enhancement surrounding a hypointense core.
Specialized MR imaging sequences allow further differentiation between brain abscesses and similarly appearing lesions. Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping help distinguish brain abscesses from cystic or necrotic tumors. A purulent abscess cavity typically exhibits high signal intensity on DWI but hypoattenuation on ADC mapping, consistent with diffusion restriction (▶ Fig. 79.4 and ▶ Fig. 79.5).14,15 In contrast, the cystic or necrotic portions of brain tumors typically appear hypointense on DWI with elevated ADC values. Exceptions to the DWI and ADC patterns may occur with toxoplasmosis abscesses and low-grade fibrillary astrocytomas.14
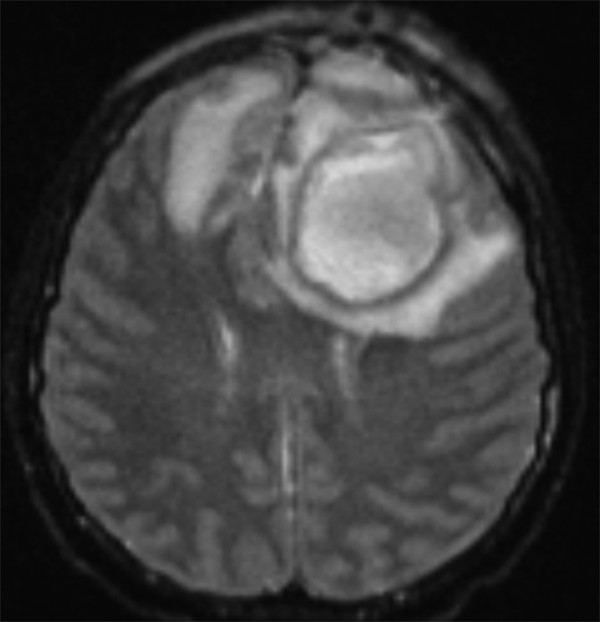
Fig. 79.4 Axial diffusion-weighted imaging sequence displaying the high-intensity inner contents of a left frontal brain abscess.
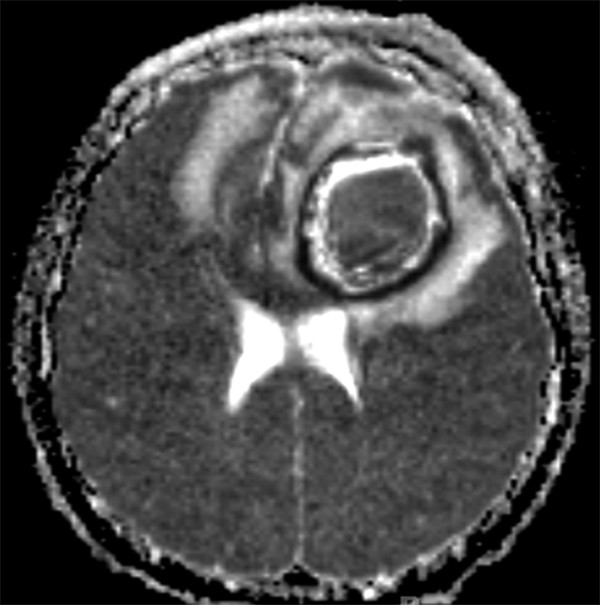
Fig. 79.5 Axial magnetic resonance imaging sequence with apparent diffusion coefficient mapping portraying low signal intensity of the central portion of the same left frontal brain abscess shown in ▶ Fig. 79.4.
MR spectroscopy may also help narrow the differential diagnosis between cystic neoplasms, necrotic tumor, and purulent collections.2 The existence of resonance peaks for succinate, acetate, lactate, and amino acids (such as valine and leucine) within the central cavity suggests the presence of a brain abscess.16 In contrast, cystic or necrotic tumors may exhibit a resonance peak only for lactate.17 MR spectroscopy combined with DWI and ADC mapping supplement conventional MR imaging findings in the diagnosis of pediatric brain abscesses.
Laboratory Studies
Multiple laboratory markers indicate the presence of infection in the setting of an intracranial abscess. These include peripheral leukocytosis and elevation of the C-reactive protein level and erythrocyte sedimentation rate. Lumbar punctures to acquire cerebrospinal fluid (CSF) are often contraindicated or provide a low yield in the setting of intraparenchymal abscesses.1,4,6 The CSF may show mild pleocytosis and an elevated protein level but typically exhibits a normal glucose concentration. Except with ventriculitis, the CSF Gram stain and cultures remain bland and sterile.6 Occasionally, culture data from blood or a contiguous site of infection suggest the culprit microorganism. However, blood cultures produce positive results in only a minority of cases.4
Additional Studies
In addition to defining an intraparenchymal abscess, the diagnostic work-up should search for predisposing factors. Concern for related sinus infections should prompt appropriate diagnostic imaging of the maxillary, ethmoid, frontal, and sphenoid sinuses (▶ Fig. 79.6). Because of the prevalence of cardiac anomalies and endocarditis in this population, echocardiography is an important tool in the diagnostic armamentarium. A thorough dental evaluation in children with brain abscesses may also reveal a surreptitious site of infection.6 In the absence of cardiac defects or obvious sites of contiguous infection, an evaluation for pulmonary infections or immunodeficiency may be valuable.
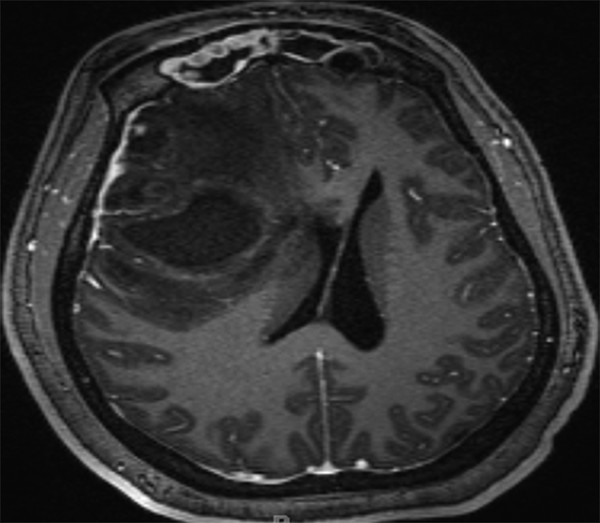
Fig. 79.6 Axial T1-weighted MR imaging sequence with gadolinium administration showing a right frontal intraparenchymal abscess with additional findings of bilateral frontal sinusitis and abnormal contrast enhancement.
79.1.9 Treatment
Indications for Surgical Treatment
Like other intracranial lesions, brain abscesses warrant surgical consideration based on size, location, mass effect, and the patient’s neurologic condition. In general, abscesses with a diameter larger than 2 to 3 cm deserve surgical attention because of mass effect.1,2,4,6 Furthermore, infratentorial abscesses merit surgical consideration because of the limited volume of the posterior fossa and risks for obstructive hydrocephalus or brainstem compression. Surgical intervention offers advantages of lowering the ICP and reducing mass effect, critical in children presenting with poor neurologic status. Additionally, surgical sampling usually provides a definitive diagnosis and identifies the offending microorganism.1,2,4 Surgical drainage also allows greater antibiotic efficacy; some antimicrobials lose their effectiveness when encumbered by many bacteria.2,4 Certain abscesses, including those in which Nocardia, helminths, parasites, or fungi are involved, respond poorly to medical therapy alone, requiring additional surgical intervention.4
Surgical Treatment
Surgical approaches for the treatment of brain abscesses include stereotactic needle aspiration and open excision. Both methods acquire samples for culture data and potentially alleviate mass effect. However, the two approaches differ greatly in regard to operative planning and invasiveness.
Stereotactic Needle Aspiration
Operative Technique
Stereotactic needle aspiration is a minimally invasive surgical approach to sampling and draining brain abscesses. The technique may be performed freehand or with intraoperative image guidance. Following appropriate placement of a standard bur hole or minimal craniotomy, the aspirating device can be advanced slowly into the necrotic core of the abscess. Intraoperative ultrasonography may help locate the abscess in real time during surgery. The biopsy needle trajectory should be chosen to avoid major vessels and eloquent cortex when possible. In rare cases of brainstem abscesses, transcerebellar or precoronal right paramedian transfrontal approaches have been used.1,13 After the aspiration of purulent material, irrigation of the abscess cavity with antibiotic-impregnated fluid may enhance the treatment effect.1 Stereotactic aspiration may be repeated in the context of multiple, residual, or recurrent abscesses.2
Advantages and Disadvantages of Stereotactic Aspiration
Stereotactic needle aspiration of brain abscesses offers several advantages over open craniotomy. As a minimally invasive approach, this technique involves less cortical exposure and damage to surrounding tissue.4 Aspiration allows the efficient decompression of purulent material in a relatively safe manner, with generally fewer complications than open approaches.1,2 Deep-seated lesions or those within eloquent cortex can be approached readily with needle biopsy, avoiding the greater risks of surgical excision. Brainstem abscesses, although accounting for fewer than 1% of intraparenchymal abscesses, similarly are favorable targets for minimally invasive techniques.13 Stereotactic aspiration also offers a safe approach to multiple brain abscesses in disparate regions. Finally, stereotactic aspiration can be used to approach abscesses in all stages of development, including early cerebritis, with a diagnostic yield near 95%.1
Despite the numerous advantages of stereotactic aspiration, this minimally invasive technique may prove inadequate in certain settings. Aspiration may fail to decompress certain abscesses completely and may not prevent purulent reaccumulation. Suboptimal decompression may result from the high viscosity of purulent fluid or intra-abscess septa.13 Residual or recurrent abscesses may require repeated stereotactic aspiration or the consideration of more aggressive surgical intervention.
Craniotomy and Abscess Resection
Operative Technique
Open craniotomy with lesion resection is an alternative approach to treating pediatric brain abscesses. Surgical excision also may be carried out with intraoperative image guidance. Wider calvarial opening and cortical exposure allow en bloc resection of the abscess wall and inner contents. During exposure and circumferential dissection, transcortical aspiration may be used urgently in the context of herniation. Once this and anesthetic maneuvers (hyperventilation, elevation of the head of the bed, administration of mannitol) lower the ICP, attention may be shifted to the abscess wall. However, friability of the parenchyma, proximity to eloquent cortex, and hemorrhage may preclude complete capsule resection. In addition to abscess removal, surgical excision allows the extrication of foreign bodies or skull fragments. Consequently, open craniotomy is the favored approach in brain abscesses secondary to penetrating head trauma.1
Advantages of Open Craniotomy
Open craniotomy for abscess excision offers several benefits over stereotactic aspiration and nonsurgical management. The greater extent of pathologic tissue removal reduces concerns for residual mass effect postoperatively. Complete resection of the abscess capsule provides more tissue for histologic analysis, definitive therapy, and lower recurrence rates.1,3,8 Surgical excision generally offers the additional benefit of a shorter duration of antibiotic therapy postoperatively (3 to 4 weeks).4 Poor neurologic status of the patient also may necessitate more aggressive approaches up front. For instance, impending herniation may warrant open surgical intervention initially to lessen mass effect and maximize decompression.
Several types of pediatric brain abscesses, in addition to those associated with trauma, warrant strong consideration of open excision. Cerebellar abscesses may require open surgical intervention because of the confined space of the posterior fossa and concern for mass effect, obstructive hydrocephalus, or brainstem compression.1,2,4 In this context, suboccipital craniotomy allows wide posterior fossa decompression in addition to abscess resection.2,3 Multiple reports suggest lower rates of mortality and postoperative hydrocephalus with open resection than with stereotactic aspiration of cerebellar abscesses.1,3
Recurrent intraparenchymal abscesses refractory to appropriate antimicrobial therapy and aspiration also are candidates for surgical resection.2,6 Certain abscesses, including fungal infections, show a poor response to stereotactic aspiration or medical therapy alone, ultimately requiring surgical excision.1 Open craniotomy with abscess removal may be preferred for multiloculate abscesses because stereotactic aspiration may fail to drain each pocket of purulent fluid.4 Furthermore, an abscess in a location abutting the ventricular system may warrant surgical resection to prevent intraventricular spillage of the abscess contents. In the context of existing ventriculitis, open craniotomy should be performed urgently to débride infected tissue, irrigate the ventricles, and administer intraventricular antibiotics.2 Additionally, brainstem abscesses may respond well to open exposure, debulking, and the drainage of purulent material with collapse of the capsule wall under direct visualization.13
Disadvantages of Open Craniotomy
Benefits of open craniotomy must be weighed against the disadvantages, including greater invasiveness, potentially longer operative times, greater surgical risk, and greater potential morbidity. Additional factors may disfavor open surgery. For instance, abscesses within stage I of formation (early cerebritis) may lack adequate development to warrant surgical excision. Multiple or deep-seated lesions and those within eloquent cortex may preclude aggressive resection, and serious medical comorbidities may elevate the surgical risk.1
Endoscopically Assisted Abscess Evacuation
Neuroendoscopy represents an alternative or supplemental approach in many neurosurgical contexts. In the treatment of intraparenchymal abscesses, endoscopy recapitulates the minimally invasive nature of stereotactic aspiration while offering the direct visualization of abscess evacuation and antibiotic lavage (if desired).1,18 Direct inspection helps maximize the drainage of purulent fluid and allows the fenestration of septa in multiloculate abscesses.1,2,18 Despite sparse literature support showing benefit over other approaches, neuroendoscopy offers an alternative method of brain abscess evacuation deserving further attention.
Multidisciplinary Approaches
Regardless of the surgical approach used, brain abscesses should be managed with a multidisciplinary approach. Frontal lobe abscesses associated with facial infections or paranasal sinusitis can be managed by pediatric neurosurgeons and by colleagues from head and neck surgery or craniofacial plastic surgery. Similarly, temporal lobe or cerebellar abscesses extending from otitis media or mastoiditis should be approached through a collaborative effort between neurosurgery and otolaryngology.1,6 This combined approach may involve a craniotomy and mastoidectomy, either concurrently or at different times.3,19 Successful treatment of both the brain abscess and the source of infection decrease the risk for abscess recurrence.1 Collaboration should extend beyond the surgical specialties to include infectious disease specialists and pediatricians for the safe and effective management of children with brain abscesses.
Antibiotic Therapy
Complementing surgical debulking, antibiotics are an integral component of the treatment of brain abscesses. Initial pharmaceutical selections are based upon the suspected microorganisms involved and the anticipated brain penetration of antimicrobials. Following empiric treatment, the antimicrobial regimen can be tailored based on culture data. The culture data may come from intracranial or extracranial sources, as long as no compelling reasons exist to assume differing identities of the microorganisms involved.
The therapeutic regimen typically entails at least a 4- to 6-week course of intravenous antibiotics, occasionally followed by oral antibiotics for 2 to 3 months.4 Some authors argue for a longer duration of intravenous antibiotics, up to 6 to 8 weeks, depending on the clinical scenario.2,13 The presence of multiple brain abscesses or immune system deficiency often warrants longer courses of intravenous antibiotics (▶ Fig. 79.7).1 The unique setting of ventriculitis alters the treatment regimen, requiring both systemic and intrathecal antibiotic administration.1,2
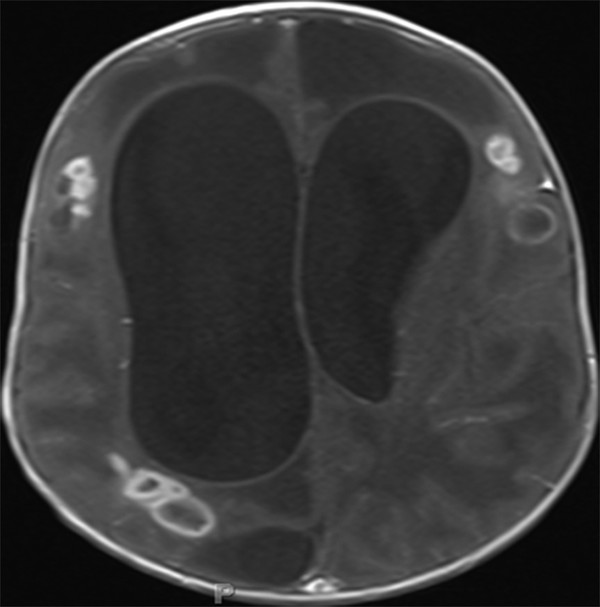
Fig. 79.7 Axial T1-weighted magnetic resonance imaging sequence with gadolinium administration depicting multiple brain abscesses in disparate locations.









