19
CHAPTER
![]()
Brain Mapping and Monitoring
Sandra Serafini, Merlise Clyde, Aatif M. Husain, and Michael Haglund
Either in the presence or in the absence of a lesion, once a patient with epilepsy is deemed a good candidate for resective surgery, they will often undergo preresection mapping to identify eloquent areas of cortex. Several techniques can be used, and the most common ones are cortical stimulation mapping (CSM) and central sulcus localization with somatosensory evoked potentials (SEP). Mapping of eloquent cortex allows the removal of maximum pathological tissue while simultaneously minimizing postoperative functional deficits in motor/sensory, language, or visuospatial abilities.
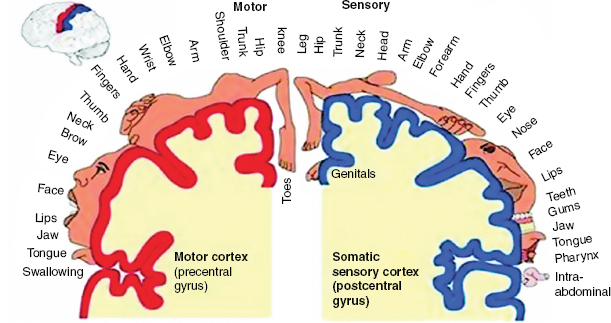
The organization of motor and sensory function was first described by Penfield and Rasmussen in a paper and schematics published in 1950 (Figure 19.1) (1). This description has been extensively relied upon to provide an expectation of the area of cortex devoted to specific motor or sensory functions, typically with good correspondence between anatomic landmarks and those respective functions in the normal population. It is not unusual, however, for motor and sensory areas to be displaced by a variety of pathologies, including epileptic foci and lesions (2,3), which introduce variability in exact functional locations from patient to patient.
Removing an epileptic focus or lesion is particularly challenging in the language-dominant hemisphere, because there is a lack of specific correspondence between anatomic landmarks and language beyond broad areas of perisylvian region such as inferior frontal, superior temporal, or inferior parietal gyri. Additional variability is likely to be introduced from an epileptic disorder, particularly with an early age of onset (4). While cortical areas necessary for motor, sensory, or language function are usually discreet (5), where a distance of only a few millimeters may be the difference between function or lack of function, the authors’ experience is also consistent with reports of occasional “transition zones” where cortical stimulation induces only occasional word-finding disruption (6). Because of this combination of individual variability, variability due to pathology, and small, discrete functional areas, it becomes necessary to map out motor, sensory, and language areas for each patient to create a tailored map from which the neurosurgeon can decide resection margins.
CORTICAL STIMULATION MAPPING METHODS
General Principles
There are two primary methods used for mapping function: (a) intraoperative and (b) extraoperative. Intraoperative mapping uses a bipolar stimulating electrode with an interelectrode distance of 5 mm (Ojemann Cortical Stimulator; Radionics, Burlington, Massachusetts). It is connected to a device that controls current amplitude, pulse-train duration for biphasic square-waves, and pulse frequency. Both the stimulator and device are shown in Figure 19.2.
Intraoperative mapping uses an “asleep-awake-asleep” method where the patient is put under deep sedation using IV propofol/remifentanil (7) while the brain is exposed via craniotomy. Once the propofol is stopped, the patient is awake within 5 to 15 minutes (8). Local anesthesia is used for scalp discomfort and IV pain medication is used for dura discomfort. Most adults and pediatric patients 12 years of age and above are suitable for intraoperative mapping when given appropriate preoperative preparation and counseling.
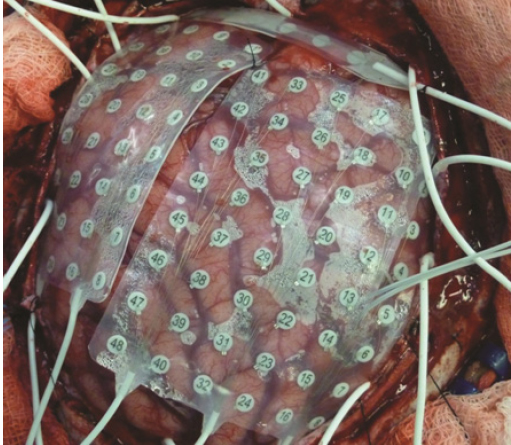
Extraoperative mapping uses a variable size grid electrode array that is placed subdurally on the surface of the cortex during a stand-alone procedure with the patient under general anesthesia. The grid is composed of a sheet (or strips) of electrodes embedded in a thin sheet of polyurethane, and within the grid are electrode discs made of a platinum alloy. Multiple grids or a grid and strip electrodes are used to aid in seizure and/or functional localization. Depth electrodes can also be placed within deeper structures of the brain to similarly aid in seizure localization. The entry point, trajectory, and depth of these electrodes are calculated by a computer to allow for precise placement. Grid and strip electrodes are 5 mm in diameter with center-to-center interelectrode distances of 1 cm (Ad-Tech, Racine, Wisconsin). Once the patient recovers from the grid placement procedure and demonstrates seizure activity, brain mapping is performed at bedside. The mapping is done by applying a small amount of electrical current through a pair of electrodes to determine if any function is located directly under the space between the electrodes. Adult patients with neocortical epilepsy, or where the epileptic focus is unclear, in addition to pediatric patients under 12 years of age are generally candidates for the two-stage procedure that accompanies extraoperative mapping. A standard grid array is shown in Figure 19.3.
FIGURE 19.1 Motor and sensory homunculus as described by Penfield and Rasmussen in 1950 (1).
![]()

FIGURE 19.2 Bipolar stimulating electrode used for intraoperative mapping (top), stimulation on an exposed cortical surface (middle), the Ojemann Cortical Stimulator used to set pulse duration, pulse rate, and current level (bottom).
![]()
FIGURE 19.3 Subdural grid arrays (4 × 8 and 6 × 8) side by side, with a 1 × 8 strip electrode placed superiorly on a brain’s exposed cortical surface.
![]()
Current amplitude begins at 2 mA and progressively increases by 1 mA to a maximum of 14 mA to determine after-discharge (AD) threshold. ADs occur when cell populations continue to fire in synchronous bursts after the driving stimulation, such as from a bipolar stimulating electrode, has ceased (see Figure 19.4). A standard stimulation procedure in adults uses a biphasic square-wave pulse of 1 millisecond at 60 Hz and a maximum train duration of 4 seconds until AD activity is evoked or current amplitude reaches 14 mA. Pediatric patients generally require longer train durations and pulse durations to evoke motor movement, sensory effects, or to interrupt a language task due to immature neurophysiologic structures and connections. For extraoperative mapping, the AD threshold must be determined for each electrode pair. Variability in stimulating currents is generally higher for pediatric patients, but can also depend on the irritability or sensitivity of the underlying cortex. Though it is suspected that epileptogenic zones are more irritable or sensitive and therefore mandate lower stimulation currents, current research has yet to demonstrate this concept clearly (9,10). For both adult and pediatric patients, it is important to remain just under the AD threshold as repeated electrical stimuli that trigger ADs can eventually generate fully generalized tonic–clonic seizures. While intravenous (IV) medication can be provided quickly to a patient to stop this seizure activity (eg, lorazepam) (9), such an event usually prevents a mapping session from continuing until the following day. Tracking the start and stop of ADs is also important as they interfere with cognitive processing. During the language task of word-finding, for example, the authors have found that ADs raise the error rate, thus providing a potential false positive that may lead to preserving a cortical site unnecessarily, potentially at the cost of seizure control. Errors made during ADs are therefore given less consideration than errors made in the absence of ADs. Conversely, if a patient is able to give accurate responses despite the occasional presence of ADs, it can be inferred that the cortical site in question is not involved with that function.
Mapping Paradigms
Motor and Sensory Paradigms
Most commonly, mapping is done to localize motor, sensory, and language function. Mapping motor function entails applying an electrical stimulus and witnessing an overt but limited motor response such as movement in the jaw, face, thumb, hand, arm, foot, or leg. The patient may also feel their tongue move or a “twitching” in the back of their throat (pharyngeal area). Sensory mapping requires the patient to communicate a sensation in a specific location after the electrical stimulus has been applied. Typical dysaesthesias are transient sensations of tingling, tickling, prickling, pricking, or burning of the tongue, jaw, face, hand, arm, foot, or leg. For young pediatric patients who exhibit a great deal of movement at baseline, a brief but distinct calming or quieting effect can be seen when they experience a dysaesthesia, at which point the patient can be prompted to describe or show where they felt the sensation.
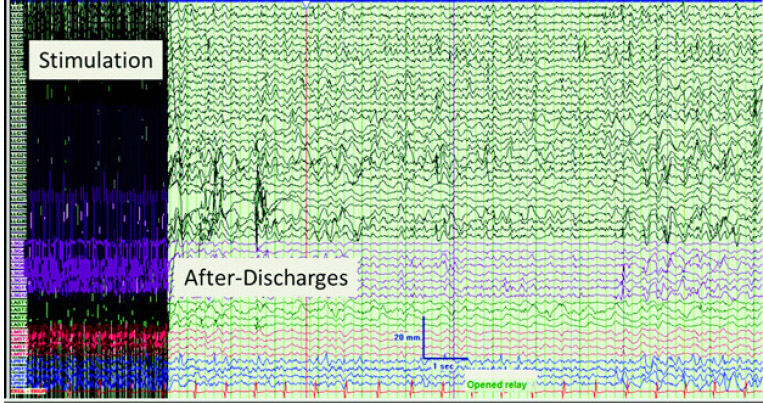
FIGURE 19.4 Electrocorticography during extraoperative mapping showing stimulation artifact and after-discharges following cessation of current application.
![]()
Speech-motor function should not be confused with language, though it is often found in the classic “Broca’s area,” ie, the operculum of the inferior frontal gyrus, but it is actually a motor function where the jaw, tongue, and face coordinate movement for articulation. It differs from motor mapping, in that the patient must perform a task that is then interrupted by the stimulation. This interruption is inferred to mean that the specific cortical site stimulated is necessary to perform the task. The tasks used for mapping this function are those that are highly overlearned, such as counting from 1 to 20, reciting the days of the week, or the months of the year. Errors noted are usually a generalized speech arrest, or the patient becomes temporarily dysarthric or apraxic, ie, they slur, stutter, or significantly lengthen the duration of the sound. Once the stimulation ceases, the patient is able to resume the task correctly. Current research from the authors is consistent with investigations from the 1980s showing that across subjects, speech-motor cortical sites are found about equally distributed in the operculum of the inferior frontal gyrus and in the ventral portion of the precentral gyrus (2,11,12). If the patient has a slow-growing lesion in this region, it is also possible for the speech-motor area to reorganize perilesionally, occasionally separating into two distinct sites around the lesion, as seen by the authors and other investigators (13).
Language Paradigms
Identifying language sites relies heavily on word-finding paradigms that use multiple input modalities, since dysnomia is a feature of virtually all aphasic syndromes; however, reading is also used as it represents an important function for resumption of postoperative academic or professional pursuits. The authors use the following paradigms: (a) visual object naming (V), (b) auditory naming (A), and (c) sentence completion (S). While visual naming (also referred to as confrontation or picture naming) has been the language task of choice since early the 1950s (14), it has become clear within the past decade that daily speech-based word-finding difficulties are seen following typical epilepsy resective procedures such as anterior temporal lobectomies when visual naming alone is used to map language (see Hamberger, 2007 and references within) (15). Word-finding errors in anterior temporal areas and the angular gyrus are best found using an auditory naming paradigm, while word-finding errors in posterior temporal, anterior supramarginal, and middle frontal areas are best found using a visual naming paradigm. Reading errors are found most often in posterior supramarginal areas with a sentence-completion task (16). It is important to note that the discovery of a language site can depend heavily on the input modality; such distinct sites prompt the authors to routinely use all three word-finding paradigms to provide a comprehensive language map before resection.
All stimuli used for language mapping with each patient must be correctly named and/or read a minimum of two separate preoperative occasions, with removal of items not named correctly. This provision reduces the false positive rate as preoperative baseline errors can strongly resemble the types of errors induced by cortical stimulation. The aim is to have a very low or no baseline error rate during cortical mapping, with the vast majority or all errors being due to the stimulation itself. The patient is instructed to provide a carrier phrase prior to naming the object (“This is a …” (visual/auditory naming) or “This says …” (sentence completion)) to ensure attentiveness to the task and a nongeneral speech arrest. Stimulation is administered during two of every three trials on average, or can alternate between stimulation and no-stimulation. It is crucial that at least one-third of the trials is conducted without stimulation to enable the calculation of a nonstimulated baseline error rate, which is used to compare against site-specific error rates and, in turn, a clinically relevant language site. A 66% error rate at a given site is usually considered clinically significant for language.
Items are chosen pseudorandomly from pictures available in the public domain (17) or within the literature (16,18), based on the preoperative abilities of each patient. A balance of items from the semantic categories of “natural” and “constructed” is given, as research indicates deficits in the naming of living stimuli and famous faces appear to be vulnerable to anterior temporal lobe resection (19–21). Such deficits are consistent with the literature on proper name anomia (ie, famous people and places) being associated with anterior temporal lobe pathology (22,23).
Bilingual Considerations
With bilingual or multilingual patients, CSM has long been used to identify cortical representations of each language. The experience of the authors is consistent with that of other investigators, who report that cortical sites may overlap across languages or be distinct to a specific language (24,25). Several factors must be taken into consideration when deciding to map a second language, the primary three being: (a) age of acquisition, (b) proficiency level of the second language, and (c) the nature of the languages (eg, alphabetic or idiographic), as each of these will influence the time devoted to mapping the second language as well as the tasks selected for mapping. For alphabetic languages, the three paradigms described earlier (visual naming, auditory naming, sentence completion) are designed with the assistance of a certified translator. For idiographic languages such as Mandarin, additional ecologically valid tasks that incorporate tonal distinction (necessary for word comprehension and production) and idiographic recognition (necessary for reading) must be included to minimize postoperative language deficits.
Error-Coding
Standard error types in word finding include the following: (a) semantic paraphasias (substituting a related word, eg, “chair” for “table”), (b) phonological paraphasias (substituting a phoneme, eg, “bar” for “car”), (c) semantic/phonological blends (eg, “plane” for “train”), (d) off-target responses, ie, unrelated to the target word, (e) no-target responses (carrier phrase given but object not named), (f) perseverations within five trials, (g) apraxic errors, (h) phonological reductions (eg, “can” for “candle”), (i) neologisms, and (j) temporal delays. Error types for reading include: (a) slow/effortful reading (apraxic), (b) syntactic errors, (c) sentence-stem additions, (d) sentence-stem omissions, and (e) mixed sentence-stem errors (additions + omissions). Occasionally, a stimulation error will carry over to a subsequent nonstimulated trial either in the presence or in the absence of ADs. Such errors count against the baseline error rate, but are given less consideration than nonstimulated error trials.
Localization and Analysis
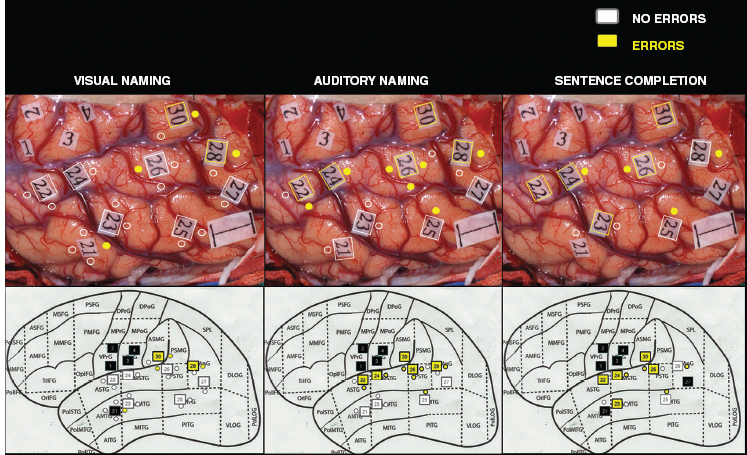
Following either intraoperative or extraoperative mapping, the neurosurgeon will place sterile 5 mm2 tags on the surface of the cortex, using a consistent numbering scheme to help localize essential areas (eg, 1–2 for motor areas, 3–5 for sensory areas, 10 for Broca’s area, even-numbered 20s for the superior temporal gyrus, odd-numbered 20s for the middle temporal gyrus, and 30–33 for the supramarginal gyrus). Intraoperative photos are taken and used for subregion localization and group analysis. An example of intraoperative photos and subregion localization is shown in Figure 19.5.
Individual analysis immediately following a mapping session uses the Fisher’s exact test to compare the trial error rates for the nonstimulated trials and the sequence of stimulated trials at a given site; rejection of the null hypothesis at a 60% error rate suggests that the location is a language site. For group analyses, the Fisher’s exact test has limitations for comparing modalities across all patients because tests are not independent across subjects, ie, each site uses the same nonstimulated set of trials to provide a baseline, and the test does not allow one to incorporate other variables that may affect error rates. To determine subregional differences across modalities, hierarchical logistic regression models are fit to the trial error indicators. Such a model incorporates the varying number of stimulation and nonstimulation trials across modalities and patients and it is not necessary for stimulation sites to be repeated across modalities within patients nor is it necessary to have an equal number of stimulation and nonstimulation trials across modalities or across patients.
FIGURE 19.5 Intraoperative naming sites in word-finding paradigms used to map language. Error-free stimulated sites (white), stimulated sites with error trials (yellow), sites not stimulated for language (black).
Abbreviations: VPrG, ventral precentral gyrus (motor strip); VPoG, ventral postcentral gyrus (sensory strip); Prefixes A=anterior, M=middle, P=posterior, Suffixes STG=superior temporal gyrus, MTG, middle temporal gyrus; ITG, inferior temporal gyrus; SMG, supramarginal gyrus; AnG, angular gyrus; 1-cm indicator shown in posterior/inferior corner of digital photos.
Source: From Ref. (16). Serafi ni S, Clyde M, Tolson M, Haglund M. Multimodality word-fi nding distinctions in cortical stimulation mapping. Neurosurgery. 2013;73:36–47.
![]()
Postoperative Language Follow-up
Studies that address postoperative language function typically assess postoperative language changes as it relates to removing or how close a resection has come to an identified site. There are very few reports on language function following the removal of visual naming sites as it was the standard of care since the 1950s to preserve those sites. The most widely accepted view of how proximate a resection can get to a visual naming language site before persistent deficits are incurred is approximately 1 cm (26), with increasing evidence that removing auditory naming sites affects postoperative naming in both visual and auditory domains (27).
Limitations
There are several advantages of using CSM to provide tailored motor, sensory, and language maps, particularly its ability to test a small, discrete area of cortex that likely mimics the effect of resection. Still, there are limitations that are recognized across epilepsy surgical centers, including: (a) the need for a high level of patient cooperation, (b) limited ability to stimulate sulcal areas, (c) the need for short yet ecologically valid language tasks, (d) low AD thresholds potentially rendering false-negative sites essential for language.
SOMATOSENSORY EVOKED POTENTIAL MAPPING
Rationale
SEP mapping is performed when the central sulcus needs to be identified. This is typically done when the suspected epileptogenic lesion lies close to motor or sensory areas. Though the central sulcus can often be identified by direct observation, when there is nearby pathology, such as a brain tumor, it can become distorted and visual identification may be inaccurate.
General Principles
The hand area has a large representation on the somatosensory and motor cortices. Consequently, median nerve stimulation is performed and cortical evoked potentials are recorded. Median nerve stimulation generates a cortical EP (N20 potential) over the somatosensory cortex. Concurrently, a positive potential is also generated over the motor cortex. The latency of this potential often occurring a little later is called the P22 potential. There are various theories that explain why a P22 potential is generated with median nerve SEP. The most commonly accepted one suggests that while most somatosensory fibers relay from the ventral postrolateral nucleus of the thalamus to the somatosensory cortex, some also relay to the motor cortex in the hand area. Hence, in addition to the N20 potential recorded from the somatosensory cortex, a P22 potential is recorded from the motor cortex. When the median nerve SEP is recorded from a series of electrodes perpendicular to the central sulcus reference to a distant site, a pseudo “phase reversal” between the N20 and P22 potentials will be noted.
Technique
Stimulation of the median nerve is performed the same way as is typically done for median nerve SEP monitoring. Instead of scalp recordings, however, a subdural strip (or sometimes grid) electrode is used for recording. The strip electrode, usually six to eight contacts, is placed perpendicular to what is assumed to be the central sulcus. Each of the strip contacts is referenced to a distal electrode, which is usually placed on the contralateral mastoid or ear. In this manner, a referential recording is obtained. A reliable response is usually seen within 50 to 100 repetitions. As noted earlier, an N20 potential is seen over the somatosensory cortex, while a P22 is seen over the motor cortex (Figures 19.6A and B). Depending on how the strip contacts are connected to the amplifier, a pseudo “phase reversal” or pseudo “positive phase reversal” will be seen. If the strip electrode contacts over the somatosensory cortex are in the lower amplifier channels (1–3), a positive phase reversal will be seen as the N20 will be toward the top of the screen and the P22 toward the bottom of the screen. If the contacts over the motor cortex are in the lower amplifier channels, the P22 will be on the top of the screen and the N20 will be below it, creating a “negative” phase reversal. The strip electrode can be moved to different locations to see if a better phase reversal can be identified (ie, electrode will be closer to the hand area). The central sulcus can also be traced from the vertex area along the lateral convexity of the cortex by progressively moving the strip electrode caudally and identifying successive sites where a phase reversal is noted.