Treatment algorithm for acute agitation.
| Medication (brand) | Recommended dose range | High-dosing recommendations | Recommended plasma concentration | Long-acting depot recommendations |
|---|---|---|---|---|
| Chlorpromazine (Thorazine) | 300–1000 mg/day | |||
| Fluphenazine (Prolixin) | 6–20 mg/day | 20–60 mg/day | 0.8–2.0 ng/ml Up to 4.0 ng/ml may be required | 2–3 week depot available. 25–100 mg/14 days |
| Haloperidol (Haldol, Serenace) | 6–40 mg/day | 20–80 mg/day Higher doses especially when failing to respond to doses up to 20 mg/day | 5–20 ng/ml Up to 30 ng/ml may be required | 4‐week depot available. 200–300 mg/28 days after loading with 200–300 mg/weekly times 3 |
| Loxapine (Loxitane) | 30–100 mg/day | |||
| Perphenazine (Trilafon) | 12–64 mg/day | |||
| Thiothixene (Navane) | 15–50 mg/day | |||
| Trifluoperazine (Stelazine) | 15–50 mg/day |
See full prescribing information for details.
| Medication (brand) | Recommended dose range | High-dosing recommendations | Recommended plasma concentration | Long-acting depot recommendations |
|---|---|---|---|---|
| Aripiprazole (Abilify) | 10–30 mg/day | Higher doses usually not more effective and possibly less effective | 4-week depot available | |
| Asenapine (Saphris) | 10–20 mg/day | High-dosing not well-studied | No depot available | |
| Clozapine (Clozaril) | 150–450 mg/day | FDA max 900 mg/day Doses >550 mg/day may require concomitant anticonvulsant administration to reduce seizure risk | No depot available | |
| Iloperidone (Fanapt) | 12–24 mg/day | High-dosing not well-studied | No depot available | |
| Lurasidone (Latuda) | 40–160 mg/day Must be taken with food. Nightly administration may improve tolerability | Efficacy of high-dosing (>160 mg/day) not well-studied | No depot available | |
| Olanzapine (Zyprexa) | 10–30 mg/day | 40–60 mg/day. Up to 90 mg/day for more difficult cases | 80–120 ng/ml | 2- and 4-week depots available |
| Paliperidone ER (Invega) | 3–12 mg/day | Max dose is generally 12 mg/day | 4-week depot available 234 mg followed after 1 week by 156 mg then continuing at 117–234 mg/28 days | |
| Quetiapine (Seroquel, SeroquelXR) | 300–750 mg/day | Up to 1800 mg /day or more for difficult cases | No depot available | |
| Risperidone (Risperdal) | 2–8 mg/day | FDA-approved up to 16 mg/day. Very high doses are usually not well-tolerated | 2-week depot available | |
| Ziprasidone (Geodon) | 80–160 mg/day Must be taken with food | Up to 360 mg/day for difficult cases | No depot available |
See full prescribing information for details.
| Medication (brand) | Recommended dose range | Dosing considerations |
|---|---|---|
| Bupropion (Wellbutrin) | 150–450 mg/day | High risk of abuse in forensic settings |
| Benzodiazepines | Various | Dose clonazepam at 0.5–2.0 mg TID and then taper as patient stabilizes. High risk of abuse in forensic settings |
| Beta blockers | Various | |
| Carbamazepine (Tegretol, generic) | 400–1200 mg/day | Target plasma concentration of 8–12 ng/ml. Recheck plasma concentration for decrease due to autoinduction 4–6 weeks after initiating. May lower plasma levels of other medications |
| Diphenhydramine (Benadryl) | 25–300 mg/day | |
| Divalproex (Depakote, DepakoteER, generic) | 750 mg/day up to 60 mg/kg/day BID or TID | May be loaded at 20–30 mg/kg, reaching steady state at around 3 days with plasma concentrations of 80–120 mcg/ml |
| Lamotrigine (Lamictal, generic) | Various | |
| Lithium (Eskalith, generic) | 900–2400 mg/day | May be initiated at 600 mg/day and titrated by 300 mg every other day to 900–1800 mg/day. Once per day dosing spares renal function. Plasma concentrations should be 0.6–1.2 mEq/l (up to 1.4 mEq/l in acute mania). Lower doses for unipolar depression (900 mg/day with serum levels of 0.6–0.9 mEq/l) |
| Oxcarbazepine (Trileptal, generic) | 1200–2400 mg/day | Less potent induction than carbamazepine, but may lower plasma levels of other medications. |
| Phenytoin (Dilantin, generic) | 300–900 mg/day | Zero-order kinetics make dosage increases result in dramatic increases in plasma concentration. Desired range is 10–20 mcg/ml. May lower plasma levels of other medications |
| SNRIs | Various | |
| SSRIs | Various | |
| TCAs | Various | Desipramine (150–300 ng/ml) and nortriptyline (50–150 ng/ml) are first‐line TCAs for impulsive aggression associated with ADHD. |
| Topiramate (Topamax) | 200–400 mg/day | |
| Trazodone (Oleptro, Desyrel, generic) | 25–600 mg /day | |
| Zolpidem (Ambien) | 5–10 mg/day |
See full prescribing information for details.

Psychotic aggression
Confirm that the patient’s violent and aggressive behaviors arise primarily from psychosis
Associated with a primary psychotic disorder (Figure 16.3) [2,3]
Associated with a major cognitive disorder (Supplemental Figure 1) [4,7,10,11]
Alzheimer’s disease [84]
Vascular dementia
Major cognitive disorder with Lewy bodies
Traumatic brain injury [12]
Antipsychotics increase the risk of mortality by 1.5- to 2-fold in elderly demented patients but may be worthwhile if alternative choices to control agitation and violence are ineffective [85,86]
Periodically test whether antipsychotic dose is required to maintain stability
It is recommended that antipsychotics be tapered and discontinued after major cognitive disorders have stabilized or progressed
Note that, although no response by weeks 4–6 of adequate to high-dose antipsychotic treatment portends a poor outcome, many patients show ongoing improvement for many weeks to months following a favorable, albeit partial, response to early treatment [87]
Some patients may require higher than cited antipsychotic plasma concentrations to achieve stabilization (Tables 16.1 and 16.2)
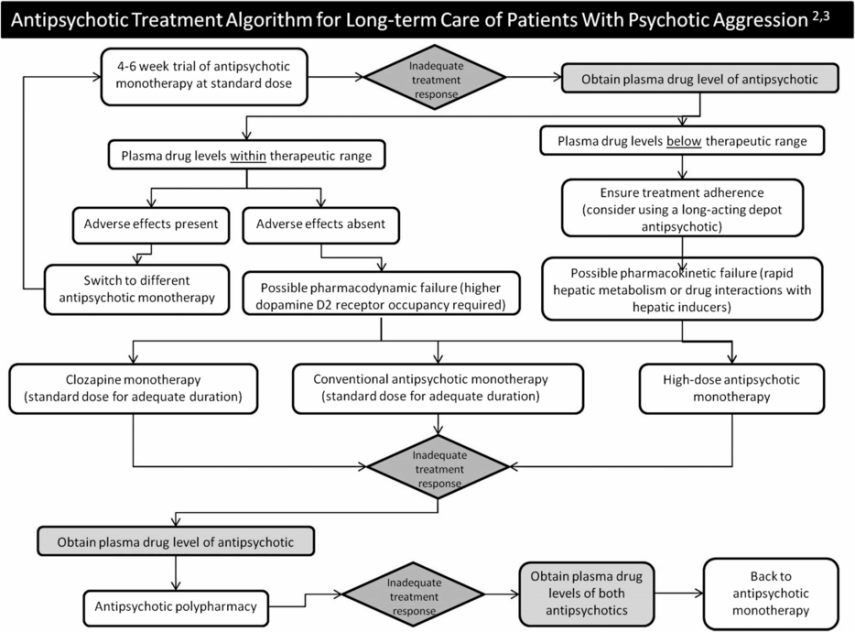
Antipsychotic treatment algorithm for long-term care of patients with psychotic aggression.
Impulsive aggression
Confirm that patient’s violent and aggressive behaviors result primarily from impulsive aggression
Characterized by reactive or emotionally charged response that has a loss of behavioral control and failure to consider consequences
Associated with
Schizophrenia spectrum disorders
Cognitive disorders [88]
ADHD (Supplemental Figure 2) [5,89]
Bipolar disorder (Supplemental Figure 3) [90–100]
Depressive disorders (Supplemental Figure 4) [101–114]
Cluster B personality disorders (Supplemental Figure 5) [115,116]
Intermittent explosive disorder (Supplemental Figure 6) [82]
PTSD (Supplemental Figure 7) [8]
TBI (Supplemental Figure 8) [12]
Strongly associated with substance use disorders
Past history of psychological trauma increases risk of impulsive aggression and is often comorbid with substance use disorders and personality disorders
For mood disorders, the goal of treatment is resolution of the mood symptoms, or improvement to the point that only one or two symptoms of mild intensity persist
For patients with mood disorders who do not achieve remission, a reasonable goal is response that entails stabilization of the patient’s safety and substantial improvement in the number, intensity, and frequency of mood (and psychotic) symptoms [121]
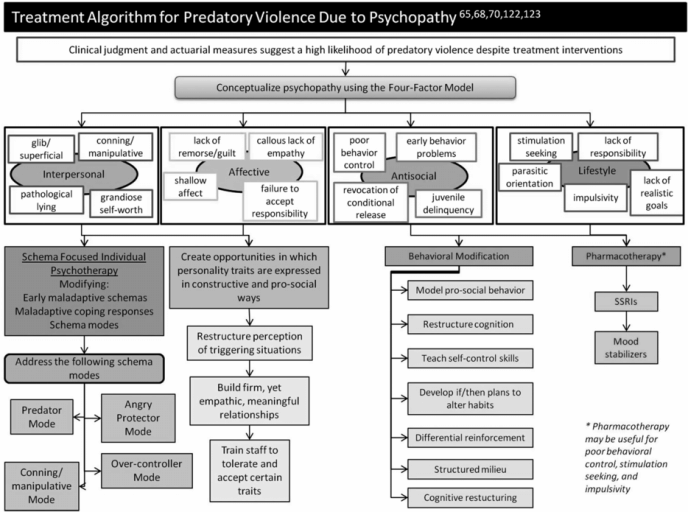
Predatory aggression
Confirm that patient’s violent and aggressive behaviors result primarily from predatory aggression
Purposeful, planned behavior that is associated with attainment of a goal
Some patients who engage in predatory acts may have the constellation of personality traits commonly known as psychopathy
Avoid countertransference reactions (Supplemental Table 3)
Determine potential reasons for predatory aggression (Supplemental Table 4) [65,68,70,122]
Provide opportunities to attain acceptable goals using social learning principles, differential reinforcement, and cognitive restructuring (Figure 16.4) [123]
Utilize the Risk–Need–Responsivity principles to determine risk level, treatment needs, and the best way to deliver and optimize treatment (Supplemental Tables 5 and 6)
Regularly evaluate the progress of predatory aggression treatment (Supplemental Table 7) [124]
Consider using mood stabilizers, SSRIs, or other antidepressants for persistent tension, explosive anger, mood swings, and impulsivity
While level of security and psychosocial interventions remain the mainstays of addressing predatory violence, preliminary data have suggested that clozapine also may reduce such aggression and violence [125]
Psychosocial Interventions
It is often the case that when treating the violently mentally ill, both medications and therapeutic interventions are needed in order to impact change
Pairing medication with appropriate psychosocial interventions can impart new coping strategies and increase medication adherence
Psychosocial interventions should also give weight to the etiology of the aggression
Once an etiology has been identified, a behavioral treatment must be further individualized based on the patient’s needs, capabilities, and other logistical limitations
Utilize the Risk–Need–Responsivity Model (Supplemental Table 6) [126–128]
Risk principle
Assessment of patient’s level of risk and contributing factors to his or her aggressive behavior
Need principle
Assessment of criminogenic needs
In this context, criminogenic needs refer to dynamic (treatable) risk factors that are correlated with criminal behavior, and when treated, reduce recidivism
Provides specific targets for treatment to reduce violence
Early antisocial behavior
Impulsive personality patterns
Negative criminal attitudes and values
Delinquent or criminal associates
Dysfunctional family relationships
Poor investment in school or work
Little involvement in legitimate leisure pursuits
Substance abuse
Individually tailor treatments to maximize the patient’s ability to learn from the interventions
Intervention is tailored toward the patient’s
Learning style
Motivation
Abilities
Strengths
Offer high-standard training on de-escalation and prevention strategies such as awareness of one’s presence (body posture), content of speech, reflective listening skills, negotiation, positive affirmation, and offering an alternative solution
Provide supportive and nonjudgmental briefing sessions to staff who are involved in incidents to discuss their subjective experience
Psychosocial interventions for psychotic aggression
General factors [129]
Good communication is essential
Multiple and coordinated treatment approaches should be used, including administrative, psychosocial, and psychotropic approaches
A sufficient dose of the selected treatment should be administered
Treatment integrity, including well-trained staff, supportive administration, and well-coordinated evaluation efforts, is vital
Treatment should be tailored to the individual
There should be a clear connection between risk assessment and treatment
Specific interventions have some evidence for efficacy in reducing violence associated with mental illness
Using cognitive behavioral methods
Behavioral modification–reinforcement
Unit and individual reinforcement
Group therapy
Cognitive therapy for psychotic symptoms
Anger management
Teaching cognitive and problem solving skills
Individual therapy
Can use various approaches
Focus on reality testing
Building alliance
Social learning [130]
Modeling by staff
Teaching cognitive and problem solving skills
Using behavioral methods
Dialectical behavior therapy (DBT) [133]
Associated with reduction in severity but not in frequency of violence in the mentally ill population
Seclusion
For up to 48 hours but not less than 4 hours
It is worth noting that anecdotal evidence suggests that some patients may respond to preventative interventions, such as time-outs, or to shorter periods of seclusion
Most experts caution against using methods that may seem punitive
Institutional approaches
Total quality management [60]
Including rewarding good behavior and changing the environment
Identifying the most aggressive individuals and targeting them for intense treatment [134]
Social structures that provide strong clinical leadership [41]
A predictable, competent, interactive, trusting environment
Intrapsychic humanism [135]
Psychosocial interventions for impulsive aggression
The goal of treatment is to increase behavioral control and decrease emotional dysregulation [136]
Psychosocial interventions for impulsive aggression with a trauma component:
Past history of psychological trauma increases risk of impulsive aggression and is often comorbid with substance use disorders and personality disorders
Treatments that incorporate trauma-informed strategies may be effective for impulsive aggression that is not responsive to other interventions [139–148]
Previous experiences of victimization often lead to difficulties in forming close relationships and ineffective coping strategies
Special emphasis on safety and therapeutic alliance
May be incorporated into many existing treatments, especially treatments for ongoing mood disorders or substance use disorders
In the case of trauma, be mindful of restraint conditions, which may re-traumatize
Exposure therapy may be useful for aggression stemming from PTSD or other traumatic experiences
More intensive and specialized treatment may be required for severely ill patients or those with chronic coping deficits or personality disorders
Psychosocial interventions for aggression due to cognitive impairment
Psychosocial interventions for aggression due to cognitive impairment
Cognitive impairment is found consistently in serious mental illness, especially schizophrenia [149–151]
Addressing complex aggressive behavior and cognitive issues should be the target of treatment
Recovery Inspired Skills Enhancement (RISE)
Multifaceted neurocognitive and social cognition training program for individuals with psychiatric disorders and severe cognitive needs and challenges
Goal of RISE is to eliminate maladaptive behaviors that interfere with an individual’s recovery process and acquisition of skills necessary for adaptive functioning
Psychosocial interventions for predatory aggression
Interventions that are tailored to the individual and provided for a sufficient amount of time can result in treatment gains [152–155]
Keeping in mind, treatment gains may be modest or non-existent
Treatments that address patients’ dynamic risk factors through psychotherapy and structured milieu interventions are most effective
Interventions to address maladaptive patterns of thinking and behavior [156]
May include theme-centered psychoeducation and process components
Modify antisocial attitudes
Improve problem solving abilities and self-regulation
Reduce resistance and impulsive lifestyles
Focus on early maladaptive schemas, schema modes, and coping responses
Seek to increase the patient’s awareness of how hostile thoughts, biases, and worldviews have contributed to his or her maladaptive behavior
If the patient is particularly psychopathic, individual therapy may be contraindicated
Milieu
Highly structured environment
Lack of access to dangerous materials
Staff having strong boundaries is crucial
Increased monitoring/externally imposed supervision
Cameras
Hospital security officers
Consider a rotation
Every interaction between the patients and a staff member should be considered an opportunity to reinforce prosocial behaviors and practice learned skills
Setting and Housing
Make all efforts to preserve patients’ self-determination, autonomy, and dignity within the treatment environment [163]
Avoid seclusion, physical restraint, and sedation when possible
Finding the right balance is key
For instance, staff should not avoid the use of restraint and seclusion to the point where the patient does not have to follow unit rules
Hospitalize patients in an enhanced treatment unit (ETU) who have [164]
Recently committed/threatened acts of violence or aggression that put others at risk of physical injury and cannot be managed in a standard treatment setting
Recurrent violent or aggressive behaviors that are unresponsive to all therapeutic interventions available in a standard treatment setting
Review attempted interventions to ensure that standard of care has been met
Communicate with treating clinicians to discuss past treatment plans
Review medications to determine if pharmacotherapy meets standard of care for the identified disorders
Review psychological assessments to determine if the relevant assessments have been attempted
Review past psychological interventions, including behavioral plans, group treatment enrollment, and individual therapy progress
A high risk of violence that cannot be contained in a standard treatment environment determined by a violence risk assessment process in conjunction with clinical judgment
The patient shows continued symptoms that increase risk for violence despite standard care
The patient refuses to engage in treatment activities
The patient refuses medication
The patient possesses prominent risk factors for violence
Examples of violence or aggression that meet criteria for ETU admission
One severe act of violence to staff or peers that causes bodily injury
Multiple acts of moderate physical violence with the potential to cause injury
A threat of significant violence (e.g., “I’m going to kill you!”) with a history of past violence
Threatening gestures or words (e.g., raised fist, slicing hand across throat) or words constituting a threat of violence
Intentional destruction of property to cause intimidation, discomfort, pain, or humiliation
Acts of sexualized violence or attempted sexual violence
Examples of behaviors that DO NOT meet criteria for ETU admission
Nuisance behavior that is disruptive but does not cause injury to peers or staff, or has little foreseeable likelihood to result in injury
Minor forms of injurious behavior unlikely to cause substantial injury or permanent damage
Sexual behavior that is consensual and does not include an aggressive or violent component
Destruction of property lacking intent or risk of personal or interpersonal harm
Inappropriate masturbation
Discharge patients from ETU who meet all of these criteria
No evident risk of aggressive or violent behavior as demonstrated by absence of
Serious rule violations
Heightened risk factors for assaultive or aggressive acts as determined by the violence risk assessment process
Threatening acts (e.g., spitting, leering, posturing to fight)
Assaultive acts
Intimidating acts
Reasonable probability that the patient will be able to maintain psychiatric stability in a less structured environment and will continue to participate in ongoing treatment activities designed to reduce violence risk
Based on documented treatment records including notes, treatment plans, and consultations
Risk assessment indicates that the patient’s current risk for aggression on a standard treatment unit or in a less structured environment is no longer elevated
Conclusion
In conclusion, the task before clinicians who treat violent mentally ill patients is great. We are challenged to help these individuals by whatever means necessary, while at the same time working within the practical restrictions of a hospital setting. The above guidelines will hopefully provide assistance with this task, and can be used as a reference. It is important to remember that many of our patients do not wish to harm others; they are simply struggling to hold themselves together, day in and day out, and it is our duty to help them achieve their highest potential. We must make every attempt to keep all those at our hospitals safe – patients and staff alike. Our concluding thought is to remember that our efforts matter; that by using science, and the best tools available, we can change the course of a life.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1092852914000376.
Disclosures
Debbi Ann Morrissette has nothing to disclose.
Marie Cugini Schur has nothing to disclose.
Jonathan Meyer has the following disclosures:
– BMS, Speaker, Speaker’s fee
– Genentech, Speaker, Speaker’s fee
– Genentech, Advisor, Honoraria
– Otsuka, Speaker, Speaker’s fee
– Sunovion, Speaker, Speaker’s fee
Eric Schwartz has nothing to disclose.
Susan Velasquez has nothing to disclose.
Darci Delgado has nothing to disclose.
Laura Dardashti has nothing to disclose.
Katherine Warburton has nothing to disclose.
George Proctor has nothing to disclose.
Michael Cummings has nothing to disclose.
Jennifer O’Day has nothing to disclose.
Charles Broderick has nothing to disclose.
Allen Azizian has nothing to disclose.
Benjamin Rose has nothing to disclose.
Shannon Bader has nothing to disclose.
Stephen M. Stahl,MD, PhD is an Adjunct Professor of Psychiatry at the University of California, San Diego School of Medicine, Honorary Visiting Senior Fellow at the University of Cambridge, UK and Director of Psychopharmacology for California Department of State Hospitals. Dr. Stahl has served as a Consultant for Astra Zeneca, Avanir, Biomarin, Envivo, Forest, Jazz, Lundbeck, Neuronetics, Noveida, Orexigen, Otsuka, PamLabs, Servier, Shire, Sunovion, Taisho, Takeda and Trius; he is a board member of RCT Logic and GenoMind; on the Speakers Bureau for Astra Zeneca, Janssen, Otsuka, Sunovion and Takeda and he has received research and/or grant support from AssureX, Eli Lilly, EnVivo, Janssen, JayMac, Jazz, Lundbeck, Mylan, Neuronetics, Novartis, Otsuka, Pamlabs, Pfizer, Roche, Shire, Sunovion, Takeda, Teva and Valeant.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree