Carbamazepine
Evan J. Fertig
Richard H. Mattson
Introduction
Carbamazepine (5H-dibenz[b,f]azepin-5-carboxamide) is one of the most widely prescribed antiepileptic drugs for the treatment of partial and generalized tonic–clonic seizures in the United States and Europe. Since 1993, nine new antiepileptic medications have been approved by the U.S. Food and Drug Administration [felbatol (Felbamate), gabapentin (Neurontin), lamotrigine (Lamictal), topiramate (Topamax), tiagabine (Gabitril), oxcarbazepine (Trileptal), levetiracetam (Keppra), zonisamide (Zonegran), and pregabalin (Lyrica). In general, these “second-generation” antiepileptic medications have better pharmacokinetic and tolerability profiles than the “first-generation” antiepileptic medications such as carbamazepine.41 Lacking, however, is any demonstration of superior efficacy. In addition, the newer agents can be significantly more expensive, and there is no clear evidence that they are more cost effective.136 Likely because of its wide availability, relative inexpensiveness, and proven clinical efficacy, carbamazepine continues to be widely used for epilepsy.
Chemical Structure, Formulation, and Monitoring
Chemical Structure and Synthesis
The development of carbamazepine, like that of many anti-epileptic drugs, came about indirectly. According to the reports of Galbraith43 and Schmutz,118 C. J. Geigy, Ltd., sought to develop a psychoactive drug in the 1950s following the introduction of the neuroleptic chlorpromazine by Rhone Poulenc. Researchers at Geigy who were seeking to develop a similarly acting tricyclic drug substituted a CH–CH for the sulfur in the chlorpromazine molecule. This change produced imipramine (Tofranil), which, unlike chlorpromazine, demonstrated antidepressant properties when tested in psychiatric patients (Fig. 1). In further efforts to find a neuroleptic, studies were made of other tricyclic carbamoyl compounds of an imino-stilbene that had been synthesized earlier in the Geigy labs by Morel.43,118 Structural changes in the molecule included the substitution at the 5 position of carboxamide for the basic side chain present in imipramine. One of these compounds (G26301) revealed even more potent anticonvulsant activity, especially in the maximal electroshock test. Early studies in animals and clinical trials confirmed its value as an antiepileptic drug. In 1957, Shindler and Lattner43,118 replaced the CH2–CH2 with a CH=CH bridge (Fig. 1) to produce carbamazepine.
Formulations
Carbamazepine is available in scored 100- and 200-mg tablets; the former are chewable and particularly useful in treating children. A suspension is also available for patients who have problems swallowing tablets or require treatment though nasogastric tubing. This liquid formulation yields peak levels about 2 hours after ingestion; peak levels are lower and occur later with the tablets.20,91,108 A slow-release formulation is available in Europe, and two for twice-daily administration are available in the United States.6,46,126,128 Use of the slow-release formulation allows avoidance of the peak and trough levels sometimes encountered when the standard tablets are used. This is important, particularly when carbamazepine is coadministered with enzyme-inducing drugs, such as phenobarbital and phenytoin.6,58,70,126,128 A recent prospective, open-label study demonstrated improved tolerability of the extended-release formulation as compared to the immediate-release formulation.38
Unfortunately, a parenteral formulation is not available because of the poor lipid solubility of carbamazepine. A soluble preparation based on a complexation of carbamazepine in cyclodextrins appears to be promising, but it has not been fully developed.37
Methods for Determination in Body Fluids
Immunoassay is the most common technique used for the determination of carbamazepine concentration in the blood. In general, this technique does not measure the metabolites of carbamazepine, some of which are biologically relevant.92 Liquid chromatographic assays have the advantage of allowing quantification of carbamazepine and its principal metabolite, carbamazepine-10,11-epoxide. Determination of free carbamazepine concentration has no additional advantage over determination of total plasma concentration.92 Carbamazepine levels in saliva have been shown to correlate predictably with plasma levels, but this technique has not been accepted into common clinical practice.19,27,68
Pharmacology
Activity in Experimental Models of Seizures/Epilepsy
The original detailed pharmacologic studies of Theobald and Kuntz127 in animal models revealed potent efficacy against seizures induced by maximal electroshock, with much less effect in preventing experimental seizures induced by pentylenetetrazol. Studies in hippocampal slice preparations revealed a reduction of burst firing in a calcium-free environment, implying a direct membrane, rather than a neurotransmitter-mediated action.55,71,85 These actions closely paralleled those of phenytoin. The promise of this new drug led to further development. Predictions of efficacy against epileptic seizures were borne out in early clinical trials of adults and children.8,10,106 Blom8 also reported that carbamazepine was effective in the treatment of trigeminal neuralgia. These early trials noted that,
unlike older available antiepileptic drugs, carbamazepine often conferred a positive “psychotropic” effect as well.32 This was attributed, at least in part, to its structural similarity to the antidepressant imipramine.
unlike older available antiepileptic drugs, carbamazepine often conferred a positive “psychotropic” effect as well.32 This was attributed, at least in part, to its structural similarity to the antidepressant imipramine.
Many open and controlled trials during the last four decades have established carbamazepine as a drug of first choice, based on its potent efficacy and overall good tolerability for the treatment of partial and tonic–clonic seizures.7,70 Europeans acquired considerable experience with the drug as a treatment for epilepsy in the 1960s. The use of carbamazepine to treat trigeminal neuralgia was approved by the U.S. Food and Drug Administration in 1968, but approval for its use in the treatment of epilepsy was delayed until 1974 because of reports of aplastic anemia. Although the initial reports of studies in children were favorable,106 carbamazepine was not approved for treatment of children in the United States for more than another 20 years.
Mechanism of Action
Confirmation of the neural membrane effect came from the very important studies by McLean and MacDonald,85 which indicated an effect at the sodium channel. They found that carbamazepine, like phenytoin, markedly reduced sustained high-frequency repetitive neuronal firing at clinically relevant concentrations. The effect was voltage and use dependent. This combination of characteristics led these and other investigators to conclude that the drug acted at the sodium channel in the “inactive state.”71 This mechanism explained why seizures with high-frequency discharge would be suppressed, whereas normal neuronal firing at lower rates would be unaffected by the drug, an observation quite in keeping with clinical effects at the usual dose.
Many other possible mechanisms have been investigated, including a synaptic action on excitatory amino acid receptors, but the evidence is not persuasive that carbamazepine is active at these sites at clinically relevant concentrations. Similarly, effects on γ-aminobutyric acid (GABA), adenosine, acetylcholine, and monoamine systems have been postulated, but without convincing evidence. On the other hand, it can be inferred that carbamazepine must have other effects on the central nervous system in addition to its action on the sodium channel because the drug has somewhat different adverse effects than phenytoin and, unlike phenytoin, possesses efficacy in the treatment of endogenous depression70 and seizures associated with alcohol withdrawal.39
Clinical Pharmacokinetics
Absorption
Carbamazepine is a member of the family of iminostilbenes—tricyclic structures that are extremely insoluble in water. The partition coefficient of carbamazepine is 58 in the octanol/aqueous system.37 Absorption is relatively complete in human studies, although some evidence indicates that infrequent large doses are less bioavailable than are more frequently administered smaller quantities.6,91,108 Samples of both generic carbamazepine and Tegretol brand have clinically relevant decreased bioavailability when kept in a humid environment.4 The drug should be stored in a closed container in a cool, dark, dry place.20 The bioavailability can vary considerably from lot to lot of both different generic formulations as well as the innovator product (Tegretol).88,91 Because of this variable absorption, patients should continue to use the same product rather than change between different generic preparations or the brand name Tegretol.
Absorption also may change perioperatively, perhaps because slowed gastrointestinal activity during bed rest at the time of surgery for epilepsy or other conditions may be associated with a transient drop in blood levels and loss of seizure control, which is then followed by toxic peak levels caused by a large reabsorption occurring after activity is resumed.124 Perioperative metabolic changes with decreased clearance also probably account for delayed transient increases in blood concentrations postoperatively. Hiremath et al. found that postoperative carbamazepine blood levels directly correlated with preoperative carbamazepine blood levels, total intraoperative fentanyl dose, and body weight. A preoperative carbamazepine level >9 was a significant risk factor for the development of symptoms of clinical toxicity following surgery.53 Carbamazepine dose reduction in the immediate postoperative period should be considered for some patients. Monitoring carbamazepine blood levels is also useful at this time.74
Plasma Protein Binding and Distribution
Carbamazepine is approximately 75% protein bound, primarily to albumin. A smaller quantity is bound to α-acid glycoprotein. The free fraction may change in medical conditions that lower serum level of albumin, such as renal and hepatic disease, which may also alter the binding properties of α-acid
glycoprotein. Certain highly bound compounds, such as valproate, may displace carbamazepine and increase its free fraction. Carbamazepine is extensively distributed to body tissue and has a volume of distribution of approximately 1.5 mL/kg.91 Following an initial dose, concentrations are highest in tissues with increased blood flow, such as cerebral cortex. During long-term administration using divided doses, brain concentration is more evenly distributed. Using intracerebral extracellular microdialysis in patients with epilepsy, Scheyer et al. determined that brain concentrations closely parallel concentrations of free carbamazepine in blood in both time and quantity.116 Entry of the metabolite carbamazepine-10,11-epoxide is more gradual, but concentrations also parallel free blood levels. The less rapid entry of the epoxide most likely reflects its lower lipid solubility.
glycoprotein. Certain highly bound compounds, such as valproate, may displace carbamazepine and increase its free fraction. Carbamazepine is extensively distributed to body tissue and has a volume of distribution of approximately 1.5 mL/kg.91 Following an initial dose, concentrations are highest in tissues with increased blood flow, such as cerebral cortex. During long-term administration using divided doses, brain concentration is more evenly distributed. Using intracerebral extracellular microdialysis in patients with epilepsy, Scheyer et al. determined that brain concentrations closely parallel concentrations of free carbamazepine in blood in both time and quantity.116 Entry of the metabolite carbamazepine-10,11-epoxide is more gradual, but concentrations also parallel free blood levels. The less rapid entry of the epoxide most likely reflects its lower lipid solubility.
Metabolism and Elimination
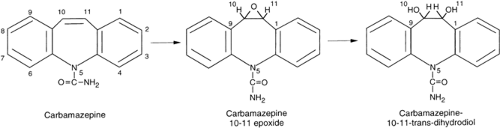
Clearance is almost entirely by hepatic metabolism. Carbamazepine is primarily oxidized by the cytochrome P450 system (CYP 3A4 isoform) to carbamazepine-10,11-epoxide. The intermediate metabolite is further hydrolyzed by microsomal epoxide hydrolase to carbamazepine-10,11-trans-dihydrodiol (Fig. 2).5,37,63,66,91 The diol and the conjugated product are eliminated by the kidneys.63,91
Carbamazepine clearance can be quite variable for several reasons. On initial administration in a single dose to volunteers, the half-life of carbamazepine is 24 to 36 hours or longer, but autoinduction during continuous administration as monotherapy approximately doubles the clearance.37,89,115 Autoinduction leads to a new steady state within a few weeks and to a half-life of about 12 to 15 hours in adults.5,36,63,66,89,114,115 Carbamazepine clearance can also be influenced by its interaction with other drugs (see later discussion).
Role of Therapeutic Drug Monitoring
The boundaries of carbamazepine’s therapeutic range vary by institution, but in general it is considered to be from 4 to 12 μg/mL. Dosing, however, should be titrated according to clinical response. Some patients with carbamazepine levels in the “therapeutic range” may have symptoms of toxicity because of elevated levels of carbamazepine-10,11-epoxide.92
The pharmacokinetic complexities of carbamazepine, especially when it is coadministered with other drugs, require awareness of factors that may inhibit or enhance its clearance as well as its pharmacologically active intermediate metabolite carbamazepine-10,11-epoxide.63,66 When an inducing or inhibiting drug is coadministered, the physician must be alert to clinical changes, and periodic monitoring of blood levels of carbamazepine is helpful. Induction may occur within a week and deinduction within a matter of days.114 If carbamazepine treatment is initiated in patients receiving oral contraceptives, antipsychotics, antiviral therapy for HIV, statins, warfarin, or cyclosporine, clearance of these drugs can be increased, with loss of efficacy and serious consequences.29,66,84,97 Inhibition of carbamazepine clearance by drugs such as erythromycin or propoxyphene may lead to increased levels and neurotoxic adverse effects within a few days. If the carbamazepine dose is lowered to compensate for the changes, reinstitution of higher doses will be needed when the interacting drug is discontinued. Levels of carbamazepine-10,11-epoxide may increase to a clinically relevant range in patients receiving carbamazepine and valproate, especially if phenobarbital or phenytoin is coadministered.63,66,74 It may be advisable to use a consistent formulation, whether generic carbamazepine or Tegretol, to minimize effects due to variable bioavailability. Periodic blood level monitoring is advisable when clinical problems arise or changes are made in coadministered drugs. All of these changes in pharmacokinetics need to be anticipated to ensure optimal control and minimal adverse effects. Measurement of complete blood cell counts and serum liver chemistries, as well as other laboratory monitoring and history and examination, especially during the first weeks and months of therapy, may help to detect medical problems.34,74,99
Efficacy
Screening tests in animals had indicated high potency in the maximal electroshock model, which commonly predicts efficacy for partial and tonic–clonic seizures. Relative lack of efficacy in the pentylenetetrazol model suggested that usefulness in generalized seizures of absence or myoclonic type would be limited. In keeping with these studies, early clinical reports8,10,106 indicated efficacy both as add-on and monotherapy in open trials. Many other open, uncontrolled trials were reported during the next decade both in adults95 and children,45 confirming efficacy for partial and generalized tonic–clonic seizures.
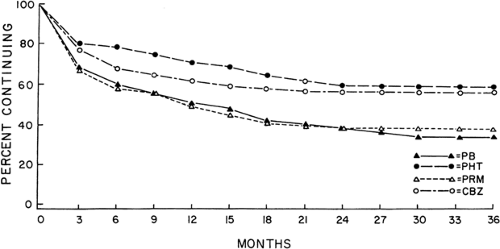 FIGURE 3. Retention (successful treatment) of 622 patients with partial seizures randomized to carbamazepine (CBZ)80, phenobarbital (PB), phenytoin (PHT), or primidone (PRM). p <.05 CBZ/PHT versus PB/PRM. |
Table 1 Patients with complex partial seizures who were seizure free for 12 months | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Use in Adults
Carbamazepine has been most widely studied for use in adults, and it has been compared to both the older and newer antiepileptic medications. In the 1970s, double-blind, controlled trials of carbamazepine as add-on therapy17,64,110,120,130 provided evidence that it was comparable in efficacy to phe-nytoin, primidone, or phenobarbital. Kosteljanetz et al.64 confirmed these results using carbamazepine and phenytoin as monotherapy; no differences were found, but the study lasted only 10 weeks and numbers were small. Ramsay et al.105 also
found carbamazepine and phenytoin to be comparably effective for partial and tonic–clonic seizures in a double-blind, randomized parallel study of monotherapy in 70 patients followed for 6 months. Many subsequent studies have confirmed these results. The largest long-term double-blind, active control comparative studies of monotherapy were conducted by the Veterans Administration (VA) Cooperative Study Groups No. 118 and No. 264. In an initial trial, 622 adult patients with partial and secondarily generalized tonic–clonic seizures were randomized to treatment, and carbamazepine was found to be equally as effective as phenobarbital, phenytoin, or primidone in controlling secondarily generalized tonic–clonic seizures in adults (Fig. 3).80 Although the results for many outcome measures of efficacy in the treatment of predominantly partial seizures (seizure frequency, rate, score, and time to first seizure)22 were equal, carbamazepine proved to be more effective than the barbiturates in providing complete control of all partial seizures, both simple and complex, at every follow-up visit for a mean duration of 3 years. This difference was statistically significant (p <.05) at the 12-month visit for patients with complex partial seizures (Table 1).
found carbamazepine and phenytoin to be comparably effective for partial and tonic–clonic seizures in a double-blind, randomized parallel study of monotherapy in 70 patients followed for 6 months. Many subsequent studies have confirmed these results. The largest long-term double-blind, active control comparative studies of monotherapy were conducted by the Veterans Administration (VA) Cooperative Study Groups No. 118 and No. 264. In an initial trial, 622 adult patients with partial and secondarily generalized tonic–clonic seizures were randomized to treatment, and carbamazepine was found to be equally as effective as phenobarbital, phenytoin, or primidone in controlling secondarily generalized tonic–clonic seizures in adults (Fig. 3).80 Although the results for many outcome measures of efficacy in the treatment of predominantly partial seizures (seizure frequency, rate, score, and time to first seizure)22 were equal, carbamazepine proved to be more effective than the barbiturates in providing complete control of all partial seizures, both simple and complex, at every follow-up visit for a mean duration of 3 years. This difference was statistically significant (p <.05) at the 12-month visit for patients with complex partial seizures (Table 1).
No difference in efficacy was observed between carbamazepine and phenytoin. In this study, phenytoin was not shown to be significantly more effective than the barbiturates. The only trial showing a difference in efficacy between carbamazepine and phenytoin was reported by Callaghan et al.15 They reported that generalized seizures were controlled completely in 73% of 37 patients treated with phenytoin and in 39% of 28 patients given carbamazepine (p <.01). The reason for the superior efficacy for phenytoin is unclear. Because all other trials have found equivalence between the two drugs, their finding may be a chance outcome. A meta-analysis was performed comparing the efficacy of carbamazepine and phenytoin monotherapy for children and adults with partial onset or generalized onset tonic–clonic seizures.131 Individual patient data were only available for analysis from 3 of the 10 studies that met their inclusion criteria for the meta-analysis,30,50,80 but nearly 60% of the individual patient data were from VA Cooperative Study Group No. 118. Not surprisingly, the meta-analysis results were congruent with this VA study: No difference was determined for efficacy between carbamazepine and phenytoin (measured in terms of 6- and 12-month seizure remission, time to first seizure) nor in time to withdrawal of treatment.
A meta-analysis was performed by the same group to examine the efficacy of carbamazepine versus phenobarbital,132 and again, of the four trials included in the analysis,30,50,80,102 the bulk of individual patient data were from the VA Cooperative Study Group No. 118. For all seizure types, time to 12-month remission and first seizure favored carbamazepine, but the results were not statistically significant because the confidence interval crossed 1. Time to withdrawal of therapy clearly favored carbamazepine, however, and was statistically significant. In subset analysis of individuals with partial seizures, phenobarbital was superior for time to first seizure. This was not a robust finding because both agents were equivalent for time to 12-month remission.
Table 2 Measures of the efficacy of carbamazepine versus valproate at 12 months in patients with complex partial seizures | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Carbamazepine is comparable in efficacy to valproate for treatment of both generalized and partial seizur-es.15,31,50,57,69,104,109 Many trials, however, have been limited by nonblinded design, short follow-up, or relatively small numbers of patients.76,79,122 In a second VA Cooperative Study, 480 adult patients were randomized to carbamazepine or valproate. Efficacy measures again included seizure count, seizure rate, seizure score, time to first seizure, and percentage of
patients who remained seizure free. Although statistically significant differences were not detected for those with predominantly tonic–clonic seizures, the trend favored carbamazepine. In fact, a later retrospective analysis of patients with only tonic–clonic seizures revealed carbamazepine to be significantly (p <.05) more effective than valproate in providing complete control at 12-month follow-up.79 For treatment of predominantly complex partial seizures, carbamazepine was significantly more effective than valproate in four of the five outcome measures but not for 100% seizure control (Table 2).82
patients who remained seizure free. Although statistically significant differences were not detected for those with predominantly tonic–clonic seizures, the trend favored carbamazepine. In fact, a later retrospective analysis of patients with only tonic–clonic seizures revealed carbamazepine to be significantly (p <.05) more effective than valproate in providing complete control at 12-month follow-up.79 For treatment of predominantly complex partial seizures, carbamazepine was significantly more effective than valproate in four of the five outcome measures but not for 100% seizure control (Table 2).82
In contrast to these reported differences in efficacy, several large long-term studies conducted in the United Kingdom and a more recent trial comparing topiramate, carbamazepine, and valproate monotherapy did not find that carbamazepine had superior efficacy. The study by Heller et al. of adults used as primary measure the percentage adult of patients entering long-term remission, a point not assessed in the VA studies.50 In the “Epiteg” trial, Richens et al.109 found that the percentage of patients entering long-term remission was similar for carbamazepine and valproate. Carbamazepine, however, had a more successful outcome in regard to time to first seizure at 1 year of follow-up. Richens et al. concluded that the apparently better outcome for carbamazepine was attributable to suboptimal dosing of valproate early in the study, and that higher doses later in the course of the treatment resulted in comparable remission rates. Another factor that might explain the different outcomes between the VA studies and those from the United Kingdom include different outcome measures.22 Only time to first seizure and complete seizure control were used in all studies. In addition, the populations were different. Patients in the UK studies had new-onset epilepsy, whereas approximately half of the patients in the VA trials had been previously treated. Patients with a history of previous treatment or undergoing long-term treatment represent a more difficult population for achieving control,83 and modest differences in efficacy may become apparent only with inclusion of more difficult cases of epilepsy. Finally, and of equal importance, differences may have been found in the VA studies because the very large number of patients in the complex partial seizure group (more than twice the number in any other study) provided the power to detect statistically significant differences. A meta-analysis comparing carbamazepine versus valproate was performed that included individual patient data from the four original studies cited earlier30,50,82,109 and an additional small pediatric study.133 Only one study was double-blinded.82 There were no overall differences in time to withdrawal of treatment, time to first seizure, or time to 12-month remission of seizures, but in subgroup analysis of patients with partial-onset seizures, carbamazepine was superior to valproate for time to first seizure and time to 12-month remission of seizures. Valproate was not, as one would expect, superior to carbamazepine for generalized-onset tonic–clonic seizures; however, the subgroup with this seizure type was small. There were also concerns that patients with partial-onset seizures may have been misclassified as generalized-onset seizures because the age of onset for this group was suspiciously late for this syndrome. In addition, outcomes for only generalized-onset tonic–clonic seizures were examined, but one would expect a greater effect of valproate for absence or myoclonic seizures. A more recent double-blind comparison of topiramate, carbamazepine, and valproate monotherapy in newly diagnosed epilepsy found no differences in efficacy in the subset with partial epilepsy.104
Table 3 Carbamazepine versus oxcarbazepine monotherapy for newly diagnosed, previously untreated epilepsy | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree