Figure 51.1. Chemical structure and main first-step metabolic pathways of oxcarbazepine and carbamazepine, and their active metabolites, MHD and CBZ-10,11-eproxide (CBZ-E).
OXC as a prodrug is rapidly and completely metabolized to R- and S-licarbazepine, which together are sometimes referred to as the monohydroxy derivative of oxcarbazepine (MHD). CBZ and OXC (and also their active metabolites—CBZ epoxide and MHD) share many known actions of antiepileptic drugs (AEDs). They produce blockade of voltage-dependent ionic membrane conductance (especially sodium, potassium, and calcium), resulting in stabilization of hyperexcited neural membranes and synaptic actions of such neurotransmitters as γ-aminobutyric acid (GABA), glutamate, purine, monoamine, N-methyl-d-aspartate, and acetylcholine receptors; the effect is diminution of propagation of synaptic impulses (2). There are subtle differences in the mechanisms of action of CBZ and OXC. For instance, MHD blocks N-type calcium channels, whereas CBZ blocks L-type (3). ESL, being converted into the active enantiomer of MHD (S-licarbazepine), is presumed to act in the same manner as does OXC. One recent study found that ESL maintains its effect on Na+-channel currents even in mice who lack the B subunit of the channel, in sharp contrast to CBZ; this effect is also seen with lacosamide, and raises the possibility that ESL may have at least some effects that are distinct from CBZ (4).
Circulating MHD exists as a racemic mixture composed of S-licarbazepine and R-licarbazepine. There appear to be potentially important differences in pharmacologic activity between these two enantiomers. For example, the affinity of R-licarbazepine appears to be about fourfold greater for voltage-gated sodium channels when in the resting state (vs. inactive state) as compared to S-licarbazepine. In addition, R-licarbazepine may block voltage-gated potassium channels (Kv7.2 currents), whereas S-licarbazepine does not. Finally, administration of eslicarbazepine was shown to inhibit acquisition of kindling in mice, whereas R-licarbazepine administration did not (5).
CARBAMAZEPINE
Absorption and Distribution
CBZ is absorbed from the gastrointestinal tract slowly, with an estimated bioavailability of about 80% to 90%. The bioavailability of the agent is similar for all formulations—that is, tablets, solution, oral suspension, chewable tablets, and extended-release tablets/capsules. However, some studies have demonstrated the advantages in reducing serum level fluctuation with controlled-release forms of CBZ. Peak plasma concentration with chronic dosing is 3 to 4 hours. CBZ is a lipophilic compound that crosses the blood–brain barrier readily and is rapidly distributed to various organs, including fetal tissues and amniotic fluid as well as breast milk (6). Pharmacokinetic parameters are shown in Table 51.1 (7–9).
Table 51.1 Pharmacokinetic Parameters of CBZ, OXC, and MHD (7–9)
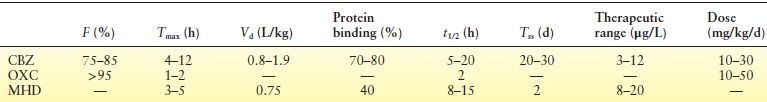
CBZ, carbamazepine; OXC, oxcarbazepine; MHD, monohydroxy derivative; F, bioavailability; Tmax, time interval between ingestion and maximum serum concentration; Vd, volume of distribution; protein binding, fraction to serum protein; t1/2, elimination half-life; Tss, steady state; therapeutic range, therapeutic range of serum concentration.
Metabolism
CBZ clearance is accomplished almost entirely via hepatic metabolism (9). The major pathways of CBZ biotransformation, consecutively or as parallel reactions, are the epoxide–diol pathway, aromatic hydroxylation, and conjugation. Metabolites from these major routes account for 80% to 90% of total urinary radioactivity. The main metabolites found in urine are due 40% to oxidation of the 10,11 double bond of the azepine rings, 25% to hydroxylation of the six-membered aromatic rings, 15% to direct N-glucuronidation at the carbamoyl side chain, and 5% to substitution of the six-membered rings with sulfur-containing groups. CBZ is oxidized by the cytochrome P450 system (CYP3A4 and CYP2C8 isoforms) to CBZ-10,11-epoxide (CBZ-E), which is considered the most important product of CBZ metabolism (see Fig. 51.1). CBZ-E is an active metabolite that may contribute to rash and other side effects associated with CBZ use. CBZ induces the activity of CYP3A4, with the metabolic clearance of CBZ-E nearly doubled in induced patients (6).
CBZ leads to autoinduction, which increases clearance (double in monotherapy), shortens serum half-life, and decreases serum concentrations. This process takes approximately 2 to 6 weeks to occur (6).
CBZ-E is hydrolyzed via the microsomal enzyme epoxide hydrolase to trans-10,11-dihydroxy-10, 11-dihydrocarbamazepine (trans-CBZ-diol). The diol is excreted in the urine and accounts for 35% of a CBZ dose and is considered to be inactive. Another, somewhat less important metabolic pathway of CBZ is the hydroxylation at different positions of the six-membered aromatic rings. The third most important step in CBZ biotransformation is conjugation reactions. CBZ may be directly conjugated with glucuronic acid. Direct N-glucuronidation of CBZ and its metabolites depends on microsomal uridine diphosphate glucuronosyltransferase (UDPGT). Additionally, CBZ and its phenolic metabolites can be conjugated with sulfuric acid (6).
Drug Interactions
CBZ has a narrow therapeutic range, and plasma concentrations are often maximized to the upper limit of tolerance. As a low-clearance drug, CBZ is sensitive to enzyme induction or inhibition, especially by the large number of agents that induce or inhibit CYP3A4 isoenzymes. Drugs that inhibit CYPA34 increase plasma concentrations of CBZ.
CBZ, like phenytoin (PHT) and phenobarbital (PB), is a broad and potent inducer of the CYP450 isozyme system including CYP3A4, CYP2C9, CYP2C19, and CYP1A2 as well as UDP-glucuronyl transferase (UGT). As a result, the metabolism of other agents, including both AEDs, and numerous other non-AED medications is increased (10). Polytherapy with CBZ can therefore result in unpredictable plasma concentrations and, potentially, clinical effect of other medications. Pharmacokinetic interactions among CBZ, OXC, and AEDs are shown in Table 51.2 (7,11).
Table 51.2 Pharmacokinetic Interactions Among CBZ, OXC, and other AEDs (7,11)
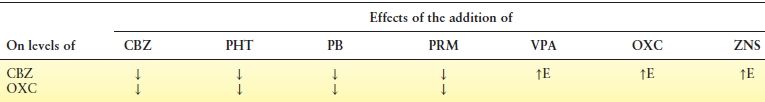
Note: Ethosuximide, felbamate, lamotrigine, gabapentin, tiagabine, pregabalin, levetiracetam, and vigabatrin addition do not affect level of OXC.
CBZ, carbamazepine; OXC, oxcarbazepine; PHT, phenytoin; PB, phenobarbital; PRM, primidone; VPA, valproate; ZNS, zonisamide; E, CBZ epoxide.
While neurologists tend to pay most attention to the effect of CBZ on other AEDs, the number of other drugs whose metabolism is induced by CBZ is vast; these include cancer chemotherapy, psychotropics, antibiotics, antithrombotic agents, antihypertensives, HMG-CoA reductase inhibitors, immunosuppressants, and many more besides (11). The number of these is so extensive, in fact, that a full discussion of these is beyond the scope of this chapter, and the reader is referred to other sources for more detail (9).
These interactions can have a profound effect on the care of other health conditions aside from the patient’s epilepsy, some of which are worth highlighting. The effectiveness of hormonal contraceptives, independent of preparation (oral, subcutaneous, intrauterine, implant, or injectable), is reduced by CBZ administration. Midcycle spotting or bleeding is a sign that ovulation has not been suppressed (10). Some practitioners believe that oral contraceptives should contain ≥50 μg of estrogen in the setting of CBZ treatment to overcome the induction, but there is a lack of good evidence to support this, so in view of the potential consequences, others take the view that OCP and CBZ should simply not be used together. Agents used for HIV infection are significantly reduced in level, and likely in efficacy, by concomitant use of enzyme-inducing AEDs, including CBZ; this is of such importance that it has generated a formal recommendation that the inducing drugs should be avoided when possible in HIV-infected patient (13). Warfarin metabolism is substantially induced by CBZ treatment, necessitating higher doses. With warfarin, and indeed all other concomitant hepatically metabolized drugs, discontinuation of CBZ will result in higher circulating levels of the other agent due to deinduction; this may result in drug toxicity. Factors pertaining to drug interaction constitute a major issue with CBZ treatment and need to be kept in mind whenever it is prescribed; the use of newer agents without such interactions avoids such complications and may be preferable in a wide range of clinical circumstances.
Efficacy
The efficacy of CBZ in patients with epilepsy was first demonstrated in the early 1960s (14). The agent continues to be a first-line treatment for patients with focal seizures.
Randomized, Monotherapy, Controlled Trials: CBZ Versus Other Agents
Most studies have demonstrated no difference in efficacy between CBZ and PHT as monotherapy for adults and children with epilepsy (14). No difference in efficacy was reported in trials comparing CBZ and PB in children. The second Veterans Administration (VA) Cooperative Study (15), a multicenter, randomized, double-blind, parallel-group trial, compared CBZ with valproate (VPA) for the treatment of 480 adults with complex partial (n = 206) or secondarily generalized (n = 274) seizures. The patient population comprised recently diagnosed, AED-naïve patients with epilepsy, as well as those who were being suboptimally treated. In patients with tonic–clonic seizures, there was no difference in efficacy between the two agents. However, CBZ appeared more efficacious than VPA for the treatment of patients with partial seizures, according to several outcome measures: number of seizures, seizure rate, seizure score, and time to first seizure. Other studies did not reveal any significant differences between CBZ and VPA in adults or children (14).
Large Trials Comparing Several AEDs with CBZ
The first VA study was a double-blind, comparative study of monotherapy with PB, PHT, primidone (PRM), and CBZ in 622 adults with partial and secondarily generalized tonic–clonic seizures (16). CBZ was found to be similarly as effective as PB, PHT, and PRM in controlling secondarily generalized tonic–clonic seizures. However, CBZ was more effective than barbiturates for the treatment of partial seizures, whether simple or complex. No difference was found between CBZ and PHT.
Other studies in the United Kingdom (14) did not demonstrate any difference between CBZ and PB, PHT, or VPA. However, the patients from the United Kingdom had been recently diagnosed with epilepsy, whereas half of the patients in the VA trials had been previously treated. Nevertheless, the large number of patients with complex partial seizures in the VA studies may provide the power to detect statistically significant differences. Because of the above-mentioned data, CBZ has been considered a first-line AED for the treatment of focal and generalized tonic–clonic seizures and is used as an active control in trials of all new compounds.
A multicentric class I study (17) of 593 elderly subjects with newly diagnosed seizures, comparing gabapentin (GBP), lamotrigine (LTG), and CBZ, concluded that there were no significant differences in the seizure-free rate at 12 months, but the main limiting factor in patient retention was adverse drug reactions. Patients taking LTG or GBP did better than those taking CBZ. Seizure control was similar among the groups. Based on the findings, the authors proposed that LTG and GBP should be considered as initial therapy for older patients with newly diagnosed seizures.
CBZ has been tested against almost all new AEDs in monotherapy trials. The majority of these studies have shown no difference in efficacy between CBZ and LTG in adults, adolescents, and children, OXC (14), or topiramate (TPM) in children and adults (18). CBZ was significantly more efficacious than were vigabatrin (VGB) (19), remacemide (20), and probably GBP (21). Some studies have suggested that GBP, LTG, VGB, and OXC are better tolerated than is CBZ.
There are several methodologic limitations in many trials, with some satisfying regulatory agencies but not necessarily guaranteeing clinical use. Most studies are either undertaken with insufficient numbers of patients to demonstrate significant differences, or else the follow-up is relatively short, considering the seizure-free period, for a true improvement in quality of life to be realized.
An important study comparing CBZ and levetiracetam did not show difference in the efficacy and effectiveness between these AEDs (22).
The available data suggest that CBZ is as effective as any of the other AEDs that have been investigated. More studies that assess the economic impact of epilepsy treatment are warranted to compare several therapies.
According to the evidence-based analysis of the AED efficacy and effectiveness as initial monotherapy for adults with focal seizures, CBZ was considered a level A recommendation (23). Based on the same guidelines, CBZ is not a level A, but a level C recommendation for elderly patients with focal epilepsy.
Adverse Events
Accurate determination of adverse events has been a limitation in several AED trials. Systematic active questioning of patients has revealed a completely different picture of a spontaneously self-reporting adverse event. Although up to 50% of patients treated with CBZ experience adverse events, only 5% to 10% need to discontinue therapy (24,25).
Neurotoxicity
Most adverse events associated with CBZ use involve the central nervous system (CNS) and are mild, transient, and dose related; severe idiosyncratic reactions occur rarely. The most common adverse events are nausea, gastrointestinal discomfort, headache, dizziness, incoordination, vertigo, sedation, diplopia or blurred vision, nystagmus, tremor, and ataxia. Adverse events are similar in children and more common in elderly patients (24,25).
As with most AEDs, CBZ may cause several psychic disturbances, including asthenia, restlessness, insomnia, agitation, anxiety, and psychotic reactions. Neuropsychological adverse events associated with nontoxic, chronic CBZ use are generally minimal. Some investigators believe that the use of a sustained-release preparation may be advantageous in both children and adults (25).
Movement disorders, including dystonia, choreoathetosis, and tics, are associated with the use of CBZ, possibly with toxic plasma levels of the agent.
Hypersensitivity Reactions
The incidence of rash with CBZ use is approximately 10% (16,26). CBZ causes the anticonvulsant hypersensitivity syndrome (AHS), characterized by fever, skin rash, and internal organ involvement (26,27). AHS is associated with the aromatic AEDs—that is, PHT, PB, PRM, CBZ, and LTG. AHS begins within 2 to 8 weeks after AED therapy initiation; the reaction usually starts with low- or high-grade fever, and over the next 1 or 2 days, a cutaneous reaction, lymphadenopathy, and pharyngitis may develop. Involvement of various internal organs may occur, resulting in hepatic, hematologic, renal, or pulmonary impairment. The most prominent manifestations are hepatitis, eosinophilia, blood dyscrasias, and nephritis. The most common cutaneous manifestation is an exanthema with or without pruritus. Rarely, severe skin reactions may occur, such as erythema multiforme, Stevens–Johnson syndrome, and toxic epidermal necrolysis (26). It is important for the management of the patient to be aware of acute cross-reactivity, which may be as high as 70% to 80% among CBZ, PHT, and PB (25,27). VPA is considered a safe, acute alternative for the treatment of patients with AHS. Systemic corticosteroids are usually required for full recovery (27).
Systemic lupus erythematosus may be induced by CBZ. Symptoms generally appear 6 to 12 months after initiation of therapy. Discontinuation of CBZ usually leads to disappearance of the symptoms. Hair loss associated with CBZ use has been reported. Myocarditis has been described as a manifestation of CBZ hypersensitivity (25).
Hematologic and Hepatic Effects
Transient leukopenia occurs within the first 3 months of treatment in 10% to 20% of patients taking CBZ. Persistent leukopenia, which is seen in 2% of patients, reverses with discontinuation of CBZ treatment (25). In the VA study, only one patient had a transient, clinically significant neutropenia (<1000 cells/mm3) associated with CBZ use, and the treatment was not discontinued (16). Isolated thrombocytopenia associated with CBZ treatment has been described at a rate of 0.9 per 100,000. Aplastic anemia and agranulocytosis have been reported in association with the use of CBZ (9,24). Data from a population-based case–control study demonstrated that the risk of developing these reactions is five to eight times greater than in the general population (9).
Hepatic enzymes may be elevated in patients receiving CBZ treatment—mostly mild elevations with no clinical significance. Rarely, CBZ hepatotoxicity can be a serious adverse event that leads to death. Cases in pediatric patients are probably less common than in adults (24). Cardiac arrhythmias have also been associated with CBZ use (25).
Metabolic Effects
Because of its potent enzyme-inducing properties, CBZ has extensive effects upon metabolism owing to the involvement of CYP450 and other similar enzymes in many endogenous metabolic processes (28). CBZ is well established to increase serum cholesterol by an average of about 25 mg/dL; it also substantially increases lipoprotein(a), doubles C-reactive protein, and may increase homocysteine as well (29–31). All of these would be expected to increase the risk of ischemic vascular disease.
Over the past several years, bone health impairment and increased risk of fractures have been associated with epilepsy and AEDs, including CBZ, both in children and in adults. There is clear evidence that CBZ decreases 25-hydroxyvitamin D levels by about 25% to 30%, accompanied by increases in markers of bone turnover (12). Because of this, many authors recommend vitamin D and calcium supplementation. Nevertheless, there is no evidence-based guidance about the efficacy of dietary supplements or the appropriate amount to be used (32,33). It is also unclear whether CBZ actually reduces bone density or increases fracture risk, with the evidence being mixed on that score (34–37).
The effect of CBZ on metabolism of testosterone, pituitary responsiveness to gonadotropin-releasing hormones, prolactin, follicle-stimulating hormone, and luteinizing hormone have been studied, although the clinical relevance of the findings has not been thoroughly elucidated (25,38). CBZ has been repeatedly shown to reduce bioactive testosterone, but not total testosterone, apparently via induction of sex hormone–binding globulin. While sizable, the clinical impact of this testosterone decrease is uncertain, with contradictory evidence regarding its effects on male sexual function (39).
Although thyroid function tests may be abnormal due to CBZ use, treated patients remain clinically euthyroid. Because of the induction effect of CBZ on the metabolism of thyroid hormones, hypothyroid patients may require higher doses of T4 to maintain euthyroid states (25).
Hyponatremia is an adverse event seen commonly by CBZ treatment. The risk for hyponatremia increases in proportion to the dose CBZ and age of the patient; it is unusual in children (25). Clinical significant hyponatremia, usually defined as sodium ≤125, is quite rare.
Weight gain is an occasional side effect associated with the use of CBZ, although it is nowhere near as pronounced as with VPA use (25)—typically in the range of 2 to 4 kg.
Teratogenic and Postnatal Effects
As with other established AEDs, CBZ exhibits teratogenic effects. CBZ exposure has also been associated with neural tube defects and major congenital malformations in monotherapy (40). Polytherapy with two or more agents significantly elevates the teratogenic risk. Despite uncertainty about the efficacy of periconceptional folate supplementation in women with epilepsy, most authors recommend its use at the same dosage as that recommended for the general population: 0.4 to 0.6 mg/day. Women taking CBZ should have prenatal diagnostic ultrasonography to detect any congenital malformations. The overall risk for CBZ causing major congenital malformation appears relatively low. Breast-feeding is considered safe for women being treated with CBZ.
A fetal AED exposure study revealed that CBZ is safer than VPA when cognitive outcomes at age 6 years are concerned (41). The North American AED Pregnancy Registry (40) assessed the safety of AEDs during pregnancy. The risk of major malformations was 3.0% for CBZ and 2.0% for lamotrigine, showing that CBZ is one of the safest AEDs during pregnancy (40). Nevertheless, when efficacy is concerned, pregnancies exposed to lamotrigine were less likely to be seizure free than were pregnancies exposed to CBZ (42).
Clinical Use
CBZ is one of the agents of choice for the treatment of structural–metabolic and unknown cause focal epilepsies, as well as for generalized tonic–clonic seizures. CBZ is available as 100-mg chewable tablets; 200-mg tablets; and 100-mg, 200-mg, and 400-mg extended-release tablets for oral administration. CBZ is also available as a 100-mg/5 mL (60 mg/mL) oral suspension (9). Doses must be adjusted individually because of great variability in different epileptic syndromes and intra- and interindividual responses.
CBZ treatment should be initiated with 100 to 200 mg/day in adults and children >12 years of age. Increments up to an initial target dose of 400 to 800 mg (10 mg/kg) in adults (60 to 80 kg) (14,15,22) and changes at weekly intervals are preferred. Risk for AHS or rash is higher with rapid titration. Newly diagnosed patients usually require lower doses than those with chronic epilepsy.
For children under 6 years of age, CBZ treatment should be initiated with 10 to 20 mg/kg/day, with a twice-daily or thrice-daily regimen. Doses can be increased at weekly intervals to achieve doses below 35 mg/kg. If a satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the therapeutic range (9).
Maintenance dosage should be adjusted to the minimum effective level, usually 400 to 1200 mg daily in adults. Dosage generally should not exceed 1000 mg daily in children 12 to 15 years of age and 1200 mg daily in patients above 15 years of age. Doses up to 1600 mg daily have been used in adults in rare instances (9). If seizures cannot be controlled, doses should be gradually increased by 100- or 200-mg increments until either control is achieved or unacceptable adverse events appear.
Although plasma level monitoring is a useful tool for the clinician, it has no definitive value. It is necessary to push the CBZ dose to the maximum clinically tolerated dose, independent of plasma level, in uncontrolled patients. Plasma level monitoring may be useful in the range of 4 to 12 mg/L (14). The dosage interval depends both on the severity of the epilepsy and on the difficulty with control. Most responsive patients, such as those newly diagnosed, need modest doses twice daily. If higher doses are necessary, however, toxicity may be avoided by taking CBZ three times per day. Two or three times per day provides similar levels, with fluctuations of 57% ± 20% and 56% ± 29%, respectively. In children, the interdose variation was 21% for patients receiving CBZ sustained-release and 41% for those treated with standard CBZ preparation. Children metabolize CBZ faster than do adults and thus may need higher doses. Elderly patients retain their sensitivity to dose-dependent autoinduction and heteroinduction by CBZ, but their metabolism rates remain considerably lower than those observed in matched controls. As a result, elderly individuals will require a lower dosage to achieve serum concentrations comparable to those found in nonelderly adults (14). In patients receiving doses that approximate the maximal tolerated doses, the use of sustained-release formulations of CBZ twice daily may minimize dose fluctuations and may help to adequately control seizures (25). On the other hand, a recent review does not confirm or refute the superiority of the sustained-release formulation with respect of seizure frequency in patients with newly diagnosed epilepsy. This study showed a trend for sustained-release CBZ to be associated with fewer adverse events when compared to immediate-release CBZ (43).
Precautions and Contraindications
CBZ should not be used in patients with a known hypersensitivity to any tricyclic antidepressant or to OXC. Use of CBZ can worsen some epileptic conditions by aggravating preexisting seizures or by leading to new seizure types, particularly absence and myoclonic seizures. An increase in the number of generalized seizures has been documented in children (14).
Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis and Stevens–Johnson syndrome, have been reported with CBZ treatment. The risk of these events is higher in patients with a particular human leukocyte antigen (HLA) allele: HLA-B* 1502. This allele occurs almost exclusively in patients with Asian ancestry. The U.S. FDA (44) has recommended genetic screening for the HLA-B* 1502 allele in patients of Asian ethnicity before starting CBZ therapy.
Aplastic anemia and agranulocytosis have been reported in association with CBZ treatment. Patient with history of adverse hematologic reaction to any drug may be particularly at risk of bone marrow depression (9).
OXCARBAZEPINE
Absorption, Distribution, and Metabolism
Orally administered OXC is rapidly and almost completely absorbed, with absorption being largely unaffected by food. As discussed earlier, the pharmacologic effect of OXC in humans is exerted predominantly through its main metabolite, MHD, which is a racemic mixture of R(-) and S(+) enantiomers. This metabolic pathway accounts for its unique pharmacokinetic and pharmacodynamic profile (45,46) (see Fig. 51.1). OXC undergoes rapid and extensive metabolism via ketoreduction to MHD. Oral absorption of OXC is extensive (>95%) and rapid. At steady state, peak concentrations of MHD are seen within about 2 to 4 hours following drug ingestion. The half-life of OXC is 1 to 3.7 hours, and the half-life of MHD is 8 to 10 hours. As a lipophilic compound, MHD is widely distributed throughout the body and easily crosses the blood–brain barrier (47). The plasma protein binding of MHD is approximately 40%, which is less than that of CBZ (70% to 90%). Steady state is achieved after three to four doses. At steady state, the pharmacokinetics of OXC is linear over the dose range of 300 to 2400 mg/day (48). After oral administration of 14C-labeled MHD, most of the dose is excreted in the urine within 6 days after dosing, <1% as unchanged drug (46). As with most AEDs, placental transfer of OXC appears to occur.
Drug Interactions
OXC exhibits no enzyme autoinduction and has a moderate potential for heteroinduction. Induction of the cytochrome P450 system is less pronounced with OXC than with CBZ (46,48). Therefore, polytherapy is much simpler with OXC. Levels of the MHD are not significantly modified by felbamate, LTG, PHT, or VPA (49). CBZ and PB may decrease levels of MHD, so dosage adjustments may be necessary (48).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








