Catheter Angiography of the Cervical Spine and Spinal Cord
Christopher G. Ullrich
Katsuyuki Nakanishi
Hironobu Nakamura
Catheter angiography of the cervical spine and spinal cord is an infrequently performed neuroradiologic procedure. Modern developments in contrast agents, catheters, image acquisition, and vascular interventional techniques have both improved the safety of these procedures and broadened their applications. This chapter reviews the indications for cervical angiography. A brief technical overview is provided, and potential complications are presented. A knowledge of cervical vascular anatomy is vital to the appropriate selection and interpretation of these procedures. Both normal and abnormal vascular findings are discussed.
INDICATIONS FOR ANGIOGRAPHY
The following are the most common indications for catheter angiography of the cervical spine and spinal cord:
To identify and characterize a primary vascular lesion. This is almost always undertaken as a secondary procedure after the possibility of such a vascular lesion has been raised either by clinical findings, such as unexplained subarachnoid hemorrhage, or because of magnetic resonance imaging (MRI) or occasionally myelographic evidence of a vascular lesion. These are rare lesions.
To delineate the vascularity and vascular blood supply of an already identified lesion or the relationship between a lesion and nearby normal blood vessels. These angiographies usually are obtained in the context of preoperative planning. It is an uncommon practice to try to distinguish benign from malignant masses using angiographic criteria. Direct percutaneous biopsy is the preferred diagnostic method in most cases involving the bony vertebrae. Spinal cord lesions are generally examined by MRI and dealt with by open surgical procedures.
In association with embolization procedures. Embolizations are most commonly performed within 48 hours of surgery to devascularize lesions and reduce operative blood loss. However, embolization can constitute the primary lesion treatment. Such embolization procedures may be either curative or may provide substantial palliation.
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have largely replaced catheter angiography for the routine evaluation of indications such as traumatic vascular injury, primary vasoocclusive diseases, and preoperative planning. Both CTA and MRA lack the isolated vascular imaging characteristics of selective catheter angiography, but image segmentation processing techniques can approximate selective angiography in many situations. Neither CTA nor MRA easily provides the temporal resolution that can be very useful in evaluating vascular malformations and shunts. The spatial resolution of CTA and MRA is very good but is below that of magnification technique catheter angiography. The combination of very good image quality, low or no x-ray exposure, ease of image acquisition, lower cost, and low patient risk inherent in CTA and MRA has greatly reduced the need and use of catheter angiography for primary diagnosis.
PATIENT PREPARATION
Careful patient preparation is a key factor in successful arteriographic examinations and for avoiding complications. A careful preprocedure medical history and examination is obtained for each patient. Present medications should be identified and appropriately maintained. Adequate hydration is continued throughout the day of the procedure. There are no absolute contraindications for arteriography. Relative contraindications include compromised renal function, multiple myeloma, sickle cell anemia, iodine allergies, hypocoagulation and hypercoagulation states, and severe hypotension or hypertension.
Compromised renal function is not uncommon, particularly in elderly patients. Serum blood urea nitrogen (BUN) and creatinine values are routinely obtained before angiography. Glomerular filtration rates (GFRs) can be estimated from serum sample testing as well and are now thought to be the best generally available measure of renal function. In patients with elevated values, hydration before and after angiography, judicious use of contrast media, and the administration of diuretic agents during and after the procedure will usually prevent subsequent renal
complications. These patients usually have serum BUN, creatinine, and GFR values checked the day after angiography to assess any changes in renal function. Patients with multiple myeloma and sickle cell disease are handled in a similar fashion to minimize the risk of renal impairment. Persons dependent on renal dialysis should undergo dialysis soon after the angiogram.
complications. These patients usually have serum BUN, creatinine, and GFR values checked the day after angiography to assess any changes in renal function. Patients with multiple myeloma and sickle cell disease are handled in a similar fashion to minimize the risk of renal impairment. Persons dependent on renal dialysis should undergo dialysis soon after the angiogram.
Iodine contrast allergy is usually a manageable problem. Oral or intravenous steroids are administered 24 hours before and after the procedure. Cimetidine and diphenhydramine are given before the procedure. This regimen is quite effective in suppressing most allergic reactions. Nonionic contrast agent is used during the procedure because it is less allergenic. Appropriate resuscitative facilities should be readily available in the angiographic suite at all times. Anesthesia standby is warranted for patients with a history of severe respiratory or cardiovascular reactions. Patients with a history of multiple major allergies are treated in a similar fashion.
Coagulopathies should be routinely corrected before angiography. Impaired coagulation is most commonly the result of medication or hepatocellular dysfunction. These patients are at increased risk for bleeding at the vascular puncture site and are less suitable candidates for embolization and surgical procedures. Hypercoagulopathy causes an increased risk of intravascular thrombus formation and unintended vascular obstruction.
Patients whose diastolic blood pressure exceeds 110 mm Hg also have an increased prevalence of hematoma formation at the vascular access site during and after arteriography. Therefore, the blood pressure should be pharmacologically controlled if possible before the procedure. Hypotensive patients are poor candidates for arteriography until their blood pressure has been stabilized at a normotensive level.
TECHNIQUE
A detailed discussion of spinal angiography technique is beyond the scope of this chapter. Readers who require more specific information are referred to the works by Abrams (1), Djindjian et al. (2,3), and Doppman et al. (4, 5 and 6).
Djindjian et al. (2,3) perform spinal angiography with the patient under general anesthesia. This helps produce technically excellent angiograms but does not allow assessment of a developing neurologic deficit during the procedure. We favor the approach of Doppman et al. (4, 5 and 6), who perform the study with the patient lightly sedated. Using this approach allows early detection of adverse events and reduces the cost of the procedure.
Angiography is performed under strict sterile conditions. Vascular access is usually via the femoral artery. Small-caliber (3, 4, or 5 Fr) catheters are often best for the selective injection of the arteries that provide the blood supply to the spine and spinal cord. A nonionic contrast agent is used because it is less neurotoxic as well as less allergenic. Heparinized saline is used to flush catheters and syringes to reduce the risk of blood-clot formation.
Two methods can be used to obtain the angiogram images: (a) conventional film-screen magnification radiography with manually produced subtraction films or (b) digital subtraction angiography (DSA). The conventional method requires the use of mechanical film changers. Intensifying screens in the changers allow rapid film exposures to be made. Multiple film images are sequentially obtained during injection of the nonionic contrast agent. To get maximum detail, an x-ray tube with a small focal spot is used and geometric magnification is performed. These images have the highest spatial resolution obtainable. Overlapping bone and soft tissue densities can obscure small but important vascular details.
Subtraction films may overcome this difficulty by displaying the contrast-filled vascular system devoid of its surrounding structures. To facilitate the film subtraction process, the first radiograph in any sequence is timed so that no contrast media are present. A reversal film, termed the mask, is prepared from this first radiograph. The subtraction film then is produced by rephotographing this mask film superimposed on the contrast angiogram film. Preexisting bone and soft tissue densities are thus “subtracted,” leaving only the contrast media-opacified vascular system visible on the resulting subtraction film. Acceptable subtraction films require exact registration between the mask and the angiogram film. Patient motion degrades the quality of the subtraction. These derived images are extremely helpful for the correct interpretation of the angiogram. Unfortunately, manual film subtraction is a slow, tedious process. Subtraction films are often not available until after the actual angiogram procedure is completed. These film-based angiographic techniques are of excellent quality but are now obsolete. The now-dominant digital imaging techniques are extensions of this work.
High-resolution intraarterial DSA is now the standard technique for angiography of the cervical spine and spinal cord. The digital image data are acquired either directly from flat panel detector plates or by analog-to-digital conversion of an image intensifier image. Compared with film, a large improvement in contrast resolution is obtained at the expense of somewhat lower spatial resolution. This reduced spatial resolution is seldom critical to clinical diagnosis.
Selective catheterization arterial DSA offers several major advantages over film-based techniques: DSA provides rapid access to both contrast angiogram and subtraction images during the actual procedure, thus shortening procedure time and potentially improving decisions made during the procedure. The superior contrast resolution of DSA allows the use of either more dilute contrast media or a smaller volume of contrast media, which improves patient comfort and lessens the risk of neurotoxicity and nephrotoxicity. Many DSA systems support vascular road mapping. This capability allows a previously obtained angiographic image to be superimposed on the active fluoroscopic image, facilitating difficult catheterizations and possibly helping to reduce catheter manipulation in small vessels, thereby decreasing the risk of direct vascular injury by the catheter or its guidewire. The primary digital images may also be postprocessed to compensate for patient motion, create dynamic video displays and threedimensional angiographic images, and so on. Networkaccessible computer storage of these DSA images and their radiographic interpretation allows immediate access to
this patient data within the entire hospital, the local medical community, and around the world as needed.
this patient data within the entire hospital, the local medical community, and around the world as needed.
Catheter angiography is an invasive procedure with infrequent but serious patient adverse events. Patient safety requires a well-trained physician and technical team that frequently performs angiographic and interventional procedures.
INTERVENTIONAL RADIOLOGY
Embolization is the most common angiographic type of interventional radiology procedure performed in the spine. Percutaneous biopsy, discectomy, and fusion procedures are not within the scope of this chapter. Indications for embolization include the following:
Preoperative lesion decompression to facilitate operative resection
Devascularization to control or avoid excessive operative blood loss
Palliation of unresectable or advanced lesions
Embolization is not indicated as an alternative to surgery for resectable lesions or in mixed arteriovenous malformations (AVMs) where there is considerable risk of damage to the normal spinal cord.
Various types of material are available for embolization procedures:
Detachable balloons
Stainless steel, platinum, or titanium coils
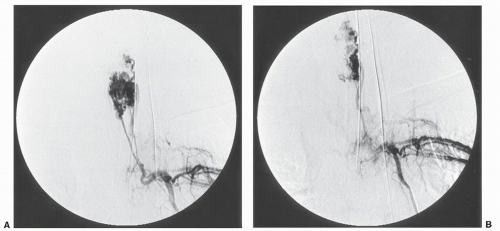
Particulates such as Gelfoam (Fig. 26.1)
Polyvinyl alcohol (PVA) foam
n-Butyl cyanoacrylate and other liquid occluding agents
Ethanol
Embosphere microspheres (Biosphere Medical, Rockland, MA)
The choice of embolic agent is based on the lesion to be treated and the desired therapeutic effect. A detailed discussion of the choice of embolization materials is beyond the scope of this chapter. When embolization is being undertaken as a primary treatment or for palliation, permanent agents such as PVA, microspheres, and coils are often used. For preoperative bleeding control, particulate Gelfoam has been extensively used in Osaka. Good control of bleeding is obtained when surgery occurs within 48 to 72 hours after Gelfoam embolization. Adverse effects on the spinal cord are rare using Gelfoam. Embolization has been ineffective for controlling venous vascular plexus bleeding in the spinal canal.
Selective catheterization chemotherapy infusion treatment for head and neck cancers has shown patient benefit. This selective infusion permits a much higher local dose of chemotherapy than could be systemically tolerated by the patient. These infusion treatments are often performed in conjunction with either surgery or radiation therapy. Similar infusion chemotherapy for primary and metastatic vertebral body tumors may become available in the future.
COMPLICATIONS OF ARTERIOGRAPHY
The best available data on angiography complications come from the cerebral angiography literature. Variations in patient populations studied and the use of different definitions for complications make direct comparison between published series difficult. Major angiographic complications include death, permanent neurologic deficit, and arterial occlusion requiring surgery or thrombolysis. Minor complications include transient neurologic deficit, hematoma, and urticaria. One well-documented series of 5,000 catheter angiography procedures by Mani et al. (7) reported a 0.16% major complication rate. Another series, reported by Bradoc and Oberson (8), described a 0.13%
major complication rate in 6,000 studies performed from 1971 to 1978 and a 0.02% major complication rate in 1,500 studies performed from 1978 to 1982. This improvement was attributed to better patient selection and increased experience with angiography. Minor complications occurred in the range of 1% to 4% in these studies. Risk factors predisposing to complications include long procedure times, large contrast doses, angiographer inexperience, large catheters, and multiple catheter exchanges. Patients with advanced cerebrovascular occlusive disease, recent stroke, subarachnoid hemorrhage, migraine, and posttraumatic and postoperative conditions tended to have more complications.
major complication rate in 6,000 studies performed from 1971 to 1978 and a 0.02% major complication rate in 1,500 studies performed from 1978 to 1982. This improvement was attributed to better patient selection and increased experience with angiography. Minor complications occurred in the range of 1% to 4% in these studies. Risk factors predisposing to complications include long procedure times, large contrast doses, angiographer inexperience, large catheters, and multiple catheter exchanges. Patients with advanced cerebrovascular occlusive disease, recent stroke, subarachnoid hemorrhage, migraine, and posttraumatic and postoperative conditions tended to have more complications.
The angiogram should be discontinued immediately whenever a neurologic complication is identified. Embolization of atherosclerotic debris and thrombi formed on the catheter or an intimal flap due to catheter trauma to the vessel are the most common causes of neurologic deficit. Contrast neurotoxicity is a much rarer cause. Anticoagulation therapy may be helpful when an intimal flap occurs, but it is of no proven benefit with atherosclerotic debris embolization. Thrombi can sometimes be successfully treated with intravascular thrombolysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree