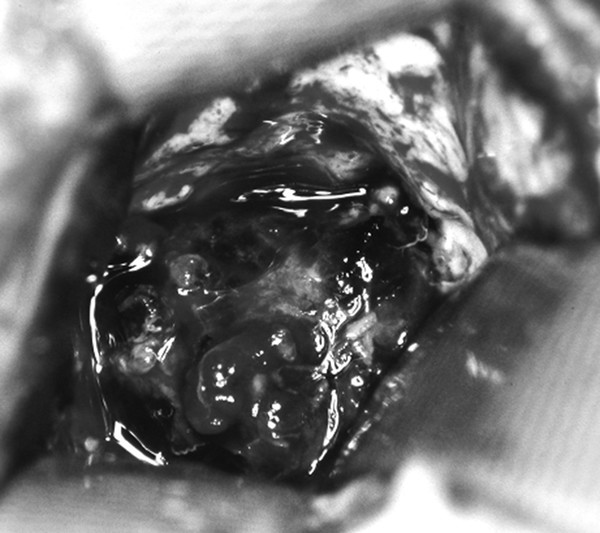
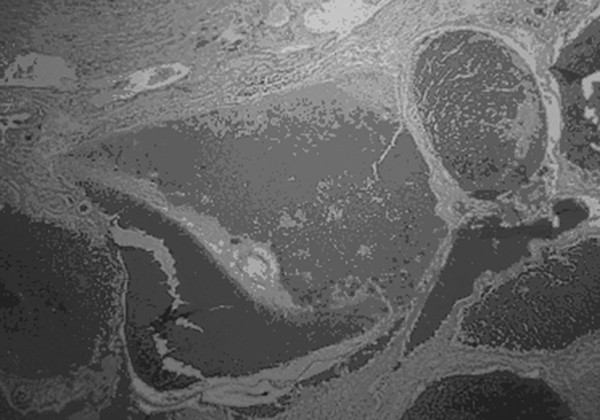
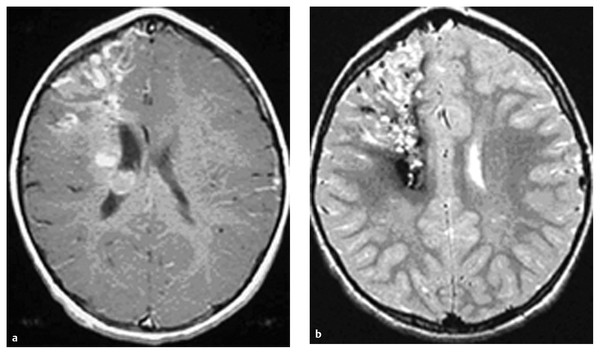
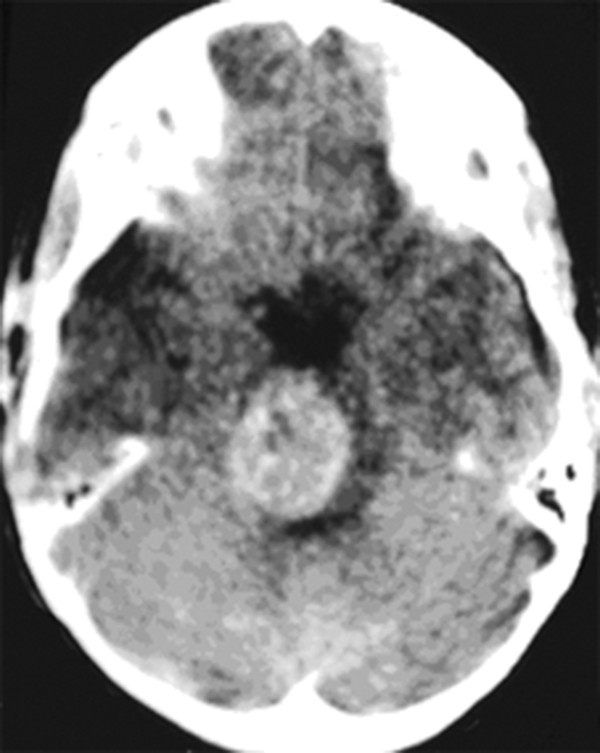
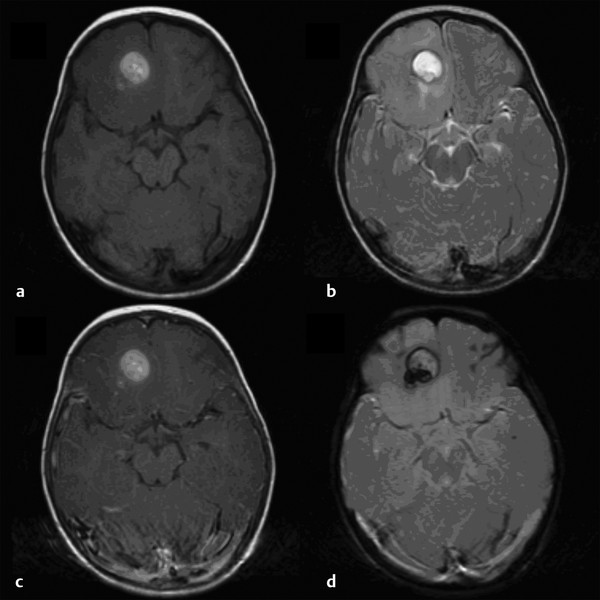
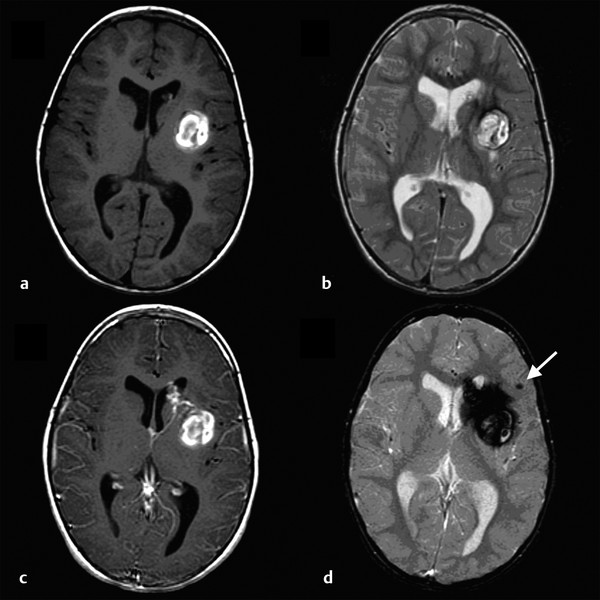
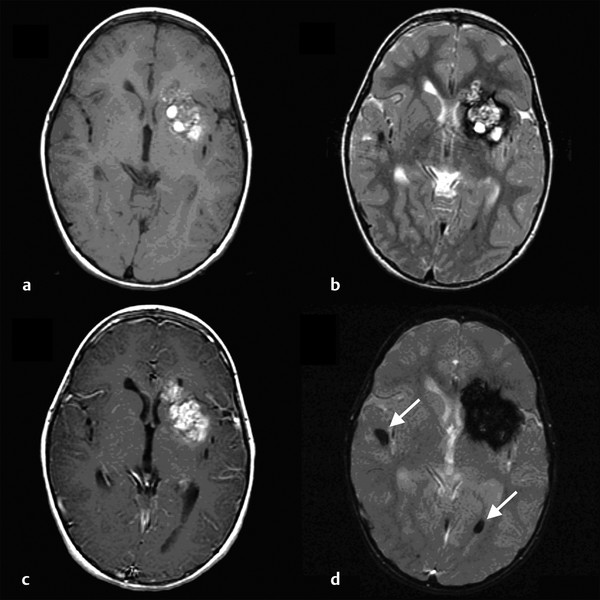
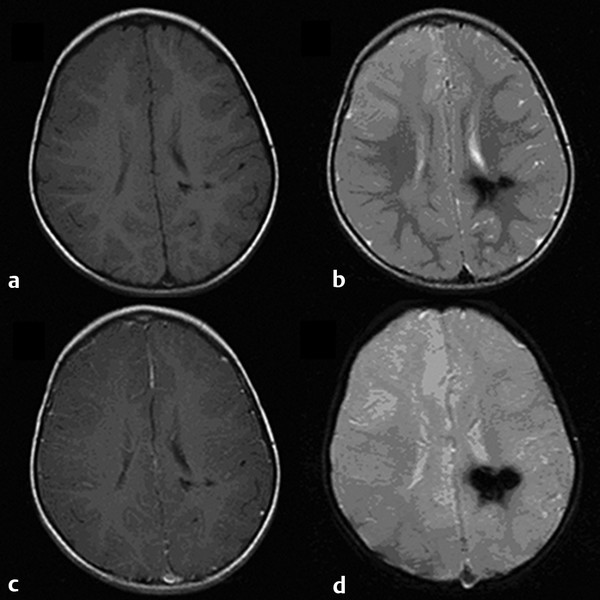
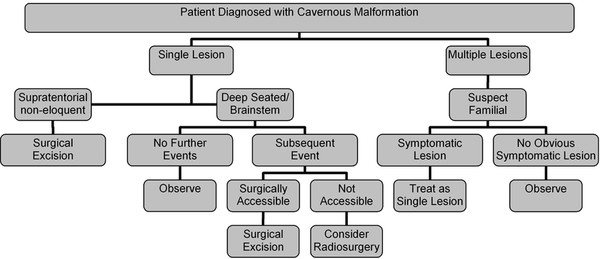
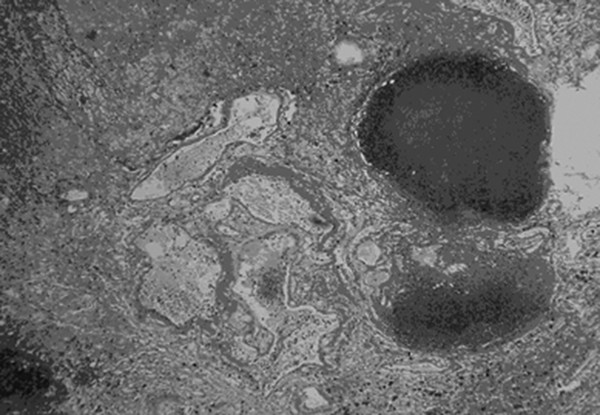
Cavernous and Venous Malformations Vascular malformations of the central nervous system (CNS) are a heterogeneous group of lesions that occur in both the brain and spinal cord. Like those in other anatomical sites, CNS vascular anomalies can be classified according to rheologic characteristics (fast-flow and slow-flow) and by channel composition (i.e., arteriovenous malformation, capillary malformation, and venous malformation) and may occur either independently or in association with syndromic conditions. Cerebral cavernous malformation (CM) consists of compact clusters of spongelike vascular spaces without intervening neural parenchyma. The nomenclature for these malformations can be confusing as they have been called cavernomas, cavernous angiomas, and cavernous hemangiomas. Venous malformation (VM) is a confluent collection of enlarged veins. Developmental venous anomaly (DVA) is a collection of small veins converging into a dilated venous trunk. Capillary malformation, usually termed capillary telangiectasia, is usually found within the substance of the brain, especially the pons or basal ganglia; it consists of an unencapsulated collection of enlarged capillaries. The focus of this chapter rests on CM, as the true venous malformations (DVA, VM, capillary telangiectasia) are not generally pathologic or causative of disease and, as a rule, should not be treated, although in certain circumstances they may cause headache or seizure disorders. In contrast, CMs can affect children through hemorrhage, seizure, focal neurologic deficits, and headache. Pathologically, CMs are a compact mass of sinusoidal vessels contiguous with one another without intervening normal parenchyma. These well-circumscribed, unencapsulated masses are identified grossly as having a purple, lobulated, “mulberry” appearance (▶ Fig. 64.1). Calcifications may be present, both grossly and microscopically (▶ Fig. 64.2). Cysts containing old hemorrhage products may be present and may help explain the controversial phenomenon of growth of these lesions, providing a substrate for neovascularization following hemorrhage. Adjacent brain may be gliotic, contain focal calcifications, and frequently is stained with hemosiderin from prior hemorrhage. Fig. 64.1 A cavernous malformation in situ following corticectomy and linear cerebrotomy. The lobulated varices contain clotted and flowing blood, and the adjacent white matter is stained orange from hemosiderin from previous hemorrhages. Fig. 64.2 Microscopic appearance of a typical cavernous malformation demonstrating thin-walled endothelial channels with little or no intervening brain tissue. Most cases are sporadic (50 to 80%), although familial variants exist, with most familial cases demonstrating multiple lesions on imaging.1,2 Very few (approximately 10%) sporadic cases will have multiple lesions. Multiple CMs can also be found in association with cranial irradiation.1,3–5 CMs are found with a prevalence of about 0.5% in autopsy studies.1,2,6,7 A diagnostic incidence of 0.43 per 100,000 people per year has been reported.8 There is no difference in sex incidence. CMs can cause symptoms of hemorrhage or progressive enlargement with mass effect. Symptoms often depend on the location of the lesion within the CNS and can include headache, seizure, and focal neurologic deficit.9–12 Seizure is the presenting symptom in 25 to 30% of cases. However, many CMs are asymptomatic and are frequently found incidentally. Initial studies that reveal CM usually consist of computed tomography (CT) or magnetic resonance (MR) imaging (▶ Fig. 64.3, ▶ Fig. 64.4, ▶ Fig. 64.5, ▶ Fig. 64.6, ▶ Fig. 64.7, ▶ Fig. 64.8). On CT, the lesions are often well circumscribed, with hyperdense, bright foci, and blood products or speckled calcification may be present.13 MR imaging usually reveals a “popcorn” appearance on T2-weighted images, with evidence of hemosiderin in surrounding tissue.17–20 Fluid–fluid levels may be present as well. An important finding is the presence of a DVA, best seen with contrast administration and commonly manifesting the “hydra head” appearance. DVA is found in the majority of pediatric CMs, and the presence of a DVA is important for surgical planning because the prevailing teaching is that this major draining vein should be preserved at resection.17–20 CMs are considered angiographically occult, meaning that they are not able to be visualized well (if at all) with catheter-based angiography.21 Fig. 64.3 Magnetic resonance imaging studies. (a) T1 with contrast enhancement and (b) T2 demonstrating deep right posterior frontal cavernous malformations with an overlying wedge-shaped cortical venous anomaly and telangiectasia. Fig. 64.4 Computed tomographic scan, unenhanced, demonstrating a massive cavernous malformation of the lateral pons and brainstem. The study shows extensive intralesional high-signal attenuation reflecting probable calcium deposition within the lesion. Fig. 64.5 Magnetic resonance imaging sequence. (a) T1, (b) T2, (c) T1 with contrast, and (d) gradient-echo images showing a type 1 lesion in the right frontal lobe. Fig. 64.6 Magnetic resonance imaging sequence. (a) T1, (b) T2, (c) T1 with contrast, and (d) gradient-echo images showing a right-sided type 2 lesion. A small type 4 lesion (arrow) is evident anterolateral to the larger type 2 lesion. In addition, a venous malformation is seen running deep to the cavernous malformation on the contrast scan. Fig. 64.7 Magnetic resonance imaging sequence. (a) T1, (b) T2, (c) T1 with contrast, and (d) gradient-echo images of the same patient as in ▶ Fig. 64.6 show a large type 2 lesion, with two small type 4 lesions (arrows) evident on the gradient-echo image. Fig. 64.8 Magnetic resonance imaging sequence. (a) T1, (b) T2, (c) T1 with contrast, and (d) gradient-echo images showing a left-sided type 3 lesion. In the setting of acute hemorrhage, other lesions, such as arteriovenous malformation (AVM), tumor, and aneurysm, should be considered. If multiple CMs are seen on imaging, the etiology may be familial or related to radiation.22 The natural history of CMs is difficult to predict. Annual rates of hemorrhage from CMs are about 3% for incidentally found lesions and 4 to 23% for hemorrhagic lesions.1,10,23–25 Some hemorrhages can be isolated, whereas others present in clusters. The usual course is an acute headache or deficit, followed by slow recovery—although often not completely back to baseline—over a few months.19,24,26–29 With the natural history as noted, some authors have advocated the early treatment of CMs in children because their anticipated long life span may favor a more aggressive approach.12,24,30,31 Symptomatic lesions are resected when feasible. Asymptomatic lesions are more controversial.32 The decision to intervene is especially difficult in the patient who presents with symptoms and has multiple lesions. If the symptoms can be localized to a single lesion that is amenable to surgical resection, then that lesion should generally be removed.1,12 Our practice is to surgically resect a single CM when it is located in non-eloquent cortex, or even in the spinal cord, if the lesion presents with symptoms, documented radiographic enlargement, or hemorrhage (usually after a minimum of about a month following hemorrhage in order to allow swelling to resolve, unless there is urgency because of significant mass effect) (▶ Fig. 64.9). For a lesion in eloquent cortex or in the brainstem, we commonly observe the lesion initially to determine if it manifests a pattern of recurrent hemorrhage that would justify the risk of surgical intervention. If hemorrhage recurs, then resection is considered. For deep lesions that are surgically inaccessible, we usually observe and treat symptomatically. We do not refer these children for radiosurgery because these malformations frequently react to such treatment with brain swelling and prolonged symptoms. Fig. 64.9 Decision pathway for the management of pediatric cavernous malformations. Referrals for genetic counseling are made for children with multiple lesions. If no lesions are symptomatic, we observe them with annual MR imaging studies. If an individual lesion grows, becomes symptomatic, or manifests new hemorrhage on imaging, then we treat following the algorithm detailed in the associated figure. The surgical management of CMs in children is similar to that in adults.1,9,32,33 In addition to the general principles of removing the entire lesion and preserving normal surrounding vasculature (especially associated DVAs), the resection of a CM may include removal of the surrounding hemosiderin ring if the lesion is cortical, associated with seizures, and in a low-risk location. In practice, the senior author (R.M.S.) rarely pursues excision of the hemosiderin as a separate strategy. In contrast, lesions in eloquent cortex, in the brainstem, or in the spinal cord should generally not have any nonlesional tissue resected in order to minimize injury to sensitive surrounding structures. At our institution, we have routinely employed frameless stereotaxy to aid in the localization of cranial lesions. This adjunct is particularly useful for deep lesions, and we have found that placement of a catheter along the planned trajectory of approach, after the dura has been opened, is helpful as a guide to the lesion during dissection. We have also found the use of intraoperative ultrasound of immense value for real-time localization and assessment of the extent of resection. The process of resection is aided by the use of nonstick bipolar forceps and the placement of patties to maintain the plane between the lesion and normal brain. We try to avoid entering the lesion, if possible, although in some areas of the brain—particularly the brainstem—this cannot be avoided. The use of gentle coagulation on the surface can often help to reduce the bulk of the lesion while preserving integrity. The use of gentle dissection around the margins of the venous outpouchings with microdissectors will help to tease the malformation out of surrounding brain tissue and help to define dissection planes. Of critical importance is careful inspection of the surgical cavity—preferably with the operating microscope—at the conclusion of the case. Small fragments of lesional tissue can easily be overlooked, especially if embedded in hemosiderin-stained tissue or when obscured by blood. Removal of the entire lesion will minimize the chance of postoperative recurrence. Radiosurgery has been used for CMs for some time, but its indications remain controversial. It is clear that CMs do not behave in the same way as AVMs that are treated with radiation. Following radiation therapy for CMs, there appears to be a temporary increase in the hemorrhage rate, in some studies up to 22.4% per patient per year34 (▶ Fig. 64.10). Following a period of 2 to 4 years, the hemorrhage rate decreases, but the results are mixed, with rates from 0.76 to 5% per patient per year. In radiosurgery series, mortality of up to 12% has been reported in brainstem studies.34–36 Fig. 64.10 Histologic section of a cavernous malformation that developed following radiation and chemotherapy for the treatment of acute lymphocytic leukemia. The malformation in the left temporal lobe had been treated with stereotactic radiosurgery 6 years previously and continued to bleed and enlarge. The section shows continued patency of many of the vascular channels of the malformation despite the radiosurgical treatment.
64.1 Pathology
64.2 Epidemiology
64.3 Presentation
64.4 Radiographic Evaluation
64.5 Differential Diagnosis
64.6 Natural History
64.7 Indications for Surgical Treatment
64.8 Surgical Treatment
64.9 Treatment Alternatives
Cavernous and Venous Malformations
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








