Chapter 38 Cerebellopontine Angle Tumors
• Comprehensive knowledge of the complex anatomy of the cerebellopontine (CP) angle is a prerequisite for achieving good surgical results. The crucial neurovascular structures should be identified as early as possible during surgery, which enables their preservation and guides subsequent operative steps. Whatever the tumor size and extension, the anatomical relationships of the cranial nerves in the area of the fundus of the internal auditory canal and in the brainstem exit/entry zone are constant.
• Most of the CP angle tumors are benign and their complete removal leads to excellent long-term outcomes. The only exception to complete tumor removal is the attempt to preserve function, such as in surgery for vestibular schwannoma in the only hearing ear.
• The major principles of CP angle tumor removal include the following: important neural structures, such as the cochlear and facial nerves, should be identified early; the tumor should be initially debulked; the dissection from the surrounding structures should be performed only after sufficient internal decompression is achieved; the dissection should always be performed in the arachnoid plane; bipolar coagulation, especially in the vicinity of a cranial nerve, should be avoided.
• Our preferred approach is the retrosigmoid approach. It is safe, relatively simple, and provides a panoramic view of the CP angle and petroclival area. Importantly, it is related to a very low procedure-related morbidity rate. The additional removal of the suprameatal tubercle provides access to tumors with extensions into Meckel’s cave, into the petroclival area, and even into the posterior cavernous sinus.
History of Cerebellopontine Angle Surgery
Tumors of the cerebellopontine (CP) angle are usually benign and their complete removal leads to the healing of the patient. However, because of the very complex anatomical structure of the area and the severity of the neurological dysfunction in case of iatrogenic damage, surgery in the CP angle has always been a challenge. The first successful complete removal of a CP angle tumor was performed in 1894 by Sir Charles Balance. The tumor was approached via a right posterior fossa craniectomy and removed with the finger inserted in an unsterile fashion between the pons and the tumor. Although the patient had facial anesthesia and complete facial palsy, he recovered from surgery and was alive for at least 18 years.1 The pathological nature of this tumor is a matter of controversy and the credit for the first removal of a vestibular schwannoma (VS) probably belongs to Thomas Annandale of Edinburgh. In 1895 he removed a tumor “the size of a pigeon’s egg” via a unilateral suboccipital craniotomy. Later, major contributions to the surgery of CP angle tumors were made by V. Horsley, von Eiselsberg, and F. Krause. Krause used for the first time faradic stimulation to differentiate the facial from the audiovestibular nerve.2 H. Cushing was the first to reduce the complication and mortality rates of VS surgery to an acceptable level by performing intracapsular tumor removal. Expectedly, the tumor recurrence rate in his series was very high. W. Dandy introduced the currently widely accepted concept of VS management. He argued that benign tumors should be removed completely in order to prevent recurrence at a later stage, even at the expense of a somewhat higher perioperative mortality rate. In the following decades, morbidity and mortality rates progressively improved with increased experience and knowledge of the normal and pathological anatomy of the CP angle, earlier detection of such tumors with the introduction of computed tomography (CT) and magnetic resonance imaging (MRI), routine use of intraoperative electrophysiological monitoring, and more reliable and safe anesthetic and operative techniques. Initial efforts at preservation of facial nerve function have expanded to preservation of hearing with increasing success. As a result of these efforts, modern CP angle surgery has been refined to a routine, safe, and low morbidity procedure.
Cerebellopontine Angle Anatomy
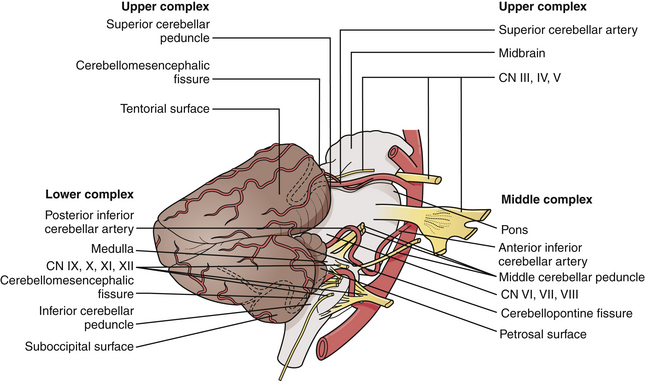
The CP angle is a triangular space located posterior to the pyramid, inferior to the tentorium, lateral to the pons, and ventral to the cerebellum.3 It is defined by the superior and inferior limbs of the CP fissure (Fig. 38.1).
The CP angle cistern is located between the anterolateral surface of the pons and cerebellum and the posterior surface of the petrous bone and contains the trigeminal, abducent, facial, and vestibulocochlear nerves, the superior cerebellar and anterior inferior cerebellar arteries, a variable number of draining veins, the flocculus of the cerebellum, and the choroid plexus that protrudes through the foramen of Luschka. The facial nerve exits from the brainstem in the lateral part of the pontomedullary sulcus, 1 to 2 mm anterior to the entry zone of the vestibulocochlear nerve. The ninth, tenth, and eleventh cranial nerves are located in the lower part of the CP angle (Fig. 38.2A and B).
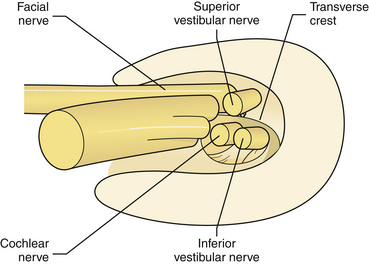
Five nerves pass through the internal auditory canal (IAC): the facial, the vestibular (superior and inferior), the cochlear, and the nervus intermedius, accompanied by the labyrinthine artery and occasionally by branches of the anterior inferior cerebellar artery (AICA) or a loop of the AICA itself.4
In the area of the fundus of the IAC, the nerves have constant location: the facial nerve occupies the anterosuperior quadrant, the cochlear nerve occupies the anteroinferior quadrant, the superior vestibular nerve is in the posterosuperior quadrant, and the inferior vestibular nerve is in the posteroinferior quadrant (Fig. 38.3). Knowledge of these anatomical relationships is of utmost importance for the surgeon because early identification of the main neurovascular structures is a prerequisite for their preservation.
Tumors of the Cerebellopontine Angle
Tumors of the CP angle account for 5% to 10% of all intracranial neoplasms.5 VSs are the most common CP angle tumor and account for 80% to 94% of them, followed by meningiomas (3-10% of CP angle tumors) and the epidermoids (2-4%). Much rarer primary tumors are schwannomas of other cranial nerves: of the trigeminal nerve, of the facial nerve, or of the caudal cranial nerves; paragangliomas, chordomas, chordosarcomas, arachnoid or neurenteric cysts, dermoid tumors, and metastases.6,7 The CP angle could be secondarily involved by tumors extending from the brainstem or fourth ventricle: gliomas, ependymomas, choroid plexus papillomas, medulloblastomas, or lymphomas. Bilateral CP angle tumors are characteristic for neurofibromatosis 2 (NF2) and are typically VSs. Rare bilateral tumors not associated with NF2 are facial nerve schwannomas, plexus papillomas, endolymphatic sac tumors, metastases, or osteomas.8
Imaging
The precise radiological diagnosis of CP angle tumors requires a systematic approach and analysis of the lesions: site of origin, location, shape and margins, density, signal intensity, and contrast enhancement characteristics.6,9 The enhancing CP angle tumors are most frequently vestibular and nonvestibular schwannomas, meningiomas, metastases and paragangliomas, chondrosarcomas, and chordomas.7 Nonenhancing extra-axial CP angle lesions may be cystic, such as the epidermoid cysts, the arachnoid cysts, and the neurenteric cyst or may contain fat (dermoid cyst, lipoma). Intrinsic brain tumors with a significant exophytic extension into the CP angle (lymphoma, hemangioblastoma, choroid plexus papilloma, ependymoma, glioma, medulloblastoma, dysembryoplastic neuroepithelial tumor) may be difficult to differentiate from an extra-axial lesion based only on their radiological characteristics.6
Bone window thin slice CT shows the bony changes of the pyramid and of the IAC and is essential for surgical planning. Erosion or dilatation of the IAC is seen in 70% to 90% of the patients with VS. Nevertheless, the diagnostic tool of choice for all CP angle tumors is MRI.7,10,11 On T1-weighted sequences VSs are isointense to slightly hypointense and on T2-weighted sequences they are hyperintense. They enhance intensely and homogeneously after contrast application, with the exception of cystic portions of the tumors. Intrameatal VSs are best visualized with gadolinium enhancement. Meningiomas are most frequently isointense to slightly hypointense to brain parenchyma on T1-weighted MRI studies. On T2-weighted MRI studies they have higher intensity than that of VS and show a homogeneous contrast enhancement. The radiological differential diagnosis between VS and CP angle meningioma is based on several criteria. Meningiomas are centered usually away from the IAC and have broad contact with the petrous bone or the tentorium (Fig. 38.4A and B). The angle between the tumor and the pyramid is obtuse. The IAC is not widened and the tumor very rarely extends into the IAC. Although secondary invasion of the IAC might be observed in 10% to 20%,12 primarily IAC meningiomas are exceedingly rare.6,13 Calcification and cystic changes are frequent findings. A tail of enhancement along the dura (the dural “tail” sign), although not pathognomonic, is visible in 60% to 72% of meningiomas. VSs are centered at the widened IAC. They form an acute angle with the posterior surface of the petrous bone and almost always extend into the IAC (Fig. 38.5). In VS, calcifications are extremely rarely found.
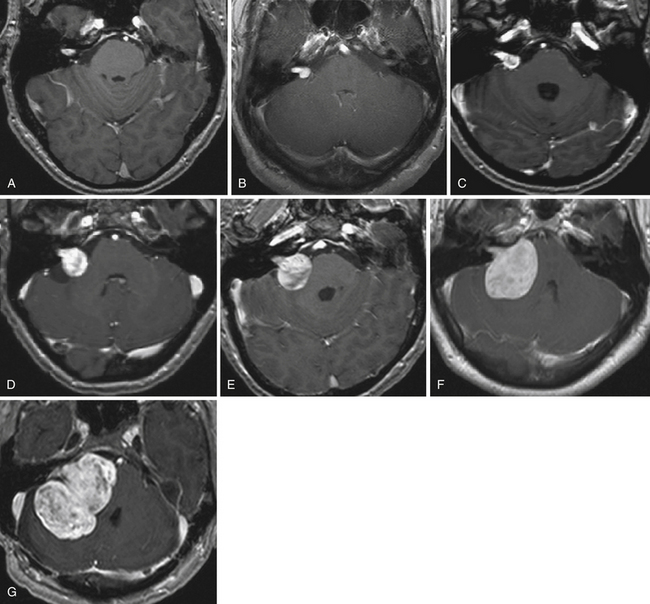
FIGURE 38.5 A to G, Stages of vestibular schwannoma growth: from grade T1 to grade T5 (T1-weighted contrast-enhanced coronal images). A, Grade T1, purely intrameatal. B, Grade T2, intraextrameatal. C, Grade T3a, filling the CPA cistern. D, Grade T3b, reaching the brainstem. E, Grade T4a, compressing the brainstem. F, Grade T4b, severe compression and dislocation of the brainstem and fourth ventricle. G, Grade T5, giant tumors with extension over the midline. See Table 38.2 for corresponding classification.
Epidermoids are hypodense on CT and in up to 25% rim calcification is observed. On T1- and T2-weighted MRI they are usually isointense to cerebrospinal fluid (CSF).6,11 The signal intensity is related to the contents of the cyst: if cholesterol predominates, the cysts are hyperintense on T1-weighted images and hypointense on T2-weighted images. Fluid-attenuated inversion recovery imaging, diffusion-weighted imaging, and CISS spin-echo MRIs are more precise imaging modalities. Because of their similar characteristics it might be difficult to differentiate epidermoid cysts from arachnoid cysts. Two of the main differences between them are the smaller mass effect caused by the arachnoid cysts and their more homogeneous signal intensity on T2-weighted images.
Surgical Approaches to the Cerebellopontine Angle
The approaches to the CP angle are either posterior (through the posterior cranial fossa) or lateral (through the petrous bone). The most popular approach is retrosigmoid suboccipital, introduced by Fedor Krause (1903) and later modified and refined by many surgeons.14 The lateral approaches involve removal of a part of the petrous bone either via a subtemporal route (e.g., the middle fossa approach, the extended middle fossa approach, or the Kawase approach) or via the mastoid (the presigmoid approach, the retrolabyrinthine petrosectomy, the translabyrinthine approach (Rudolf Panse, 1904), and the transcochlear approach).4,14
Several factors have to be considered when selecting the surgical approach: tumor type, size, and origin; extension in the CP angle or in the IAC; patient’s age and general and neurological status, especially hearing level; and surgeon’s experience and institutional tradition. The approach should adhere to the following principles: it should provide sufficient exposure of the tumor; it should not be related to significant morbidity; the neural structures should not be at increased risk; any injury to the venous outflow system should be avoided. Most of the CP angle tumors are benign and their complete removal leads to healing of the patient. The preservation and even the recovery of neurological functions and of the patient’s quality of life should always be the first priority. The only exception to complete tumor removal is the attempt to preserve function, such as in surgery for VS in the only hearing ear. Excellent results have been achieved with different operative approaches, which depend more on the individual surgeon’s experience than on the advantages or disadvantages of each particular approach. Still, an ever growing amount of evidence suggests that the goals of CP angle surgery are best achieved with the retrosigmoid suboccipital approach (RSA).3,14–16 It is safe and relatively simple and provides a panoramic view of the whole CP angle and petroclival area. Importantly, it is associated with a very low procedure-related morbidity rate. The additional removal of the suprameatal tubercle—the Samii technique, developed and introduced by the senior author in 1982—provides access to tumor extensions into Meckel’s cave, the petroclival area, and even the posterior cavernous sinus.
Retrosigmoid Approach
A drawback of this patient position is the risk of venous air embolism, paradoxical air embolism, tension pneumocephalus, or circulatory instability. However, in experienced hands these effects are not related to any lasting morbidity.17 Transesophageal echocardiography is the most specific and sensitive method in detection of air embolism but the combined monitoring of end-tidal carbon dioxide and precordial Doppler echocardiography yield similar results. If immediate measures are carried out at the first sign of venous air embolism, the related morbidity is insignificant.
Continuous neurophysiological monitoring should be performed throughout the surgery from the time of positioning of the patient to the skin closure. It includes monitoring of somatosensory evoked potentials (important during patient positioning in order to prevent spinal cord compression); electromyography of the facial nerve; and monitoring of the brainstem auditory evoked potentials in the case of preoperatively available hearing.18,19 Monitoring of the oculomotor, trochlear, abducens, and caudal cranial nerves is performed if needed according to the particular tumor extension and clinical presentation.
A slightly curved skin incision approximately 2 cm medial to the mastoid process is performed and the underlying muscles are incised in line with the skin. We make the bur hole approximately 2 to 2.5 cm below the superior nuchal line, two thirds behind and one third in front of the occipitomastoid suture. The asterion is not an absolutely reliable anatomical landmark and is variable both in the cranial-caudal plane and in the anterior-posterior plane.20 The sigmoid sinus descends along an axis defined by the mastoid tip and the squamosal-parietomastoid suture junction or over the mastoid groove. The course of the transverse sinus is more variable and the superior nuchal line gives a rough orientation of its location. The issue of whether to make a craniectomy or craniotomy is a matter of individual preference but we avoid making one-piece craniotomy due to the related high risk of injury to the underlying sinuses and the risk of tearing the dura by the craniotome. Excessive traction to the mastoid emissary vein/veins could lead to sinus laceration and increases the risk of venous air embolism. The vein should be skeletonized with a diamond drill until it is free of any bony encasement and can be safely coagulated. The lateral and superior limits of the approach are the borders of the sigmoid and of the transverse sinuses; their edges have to be exposed. Inferiorly, enough bone should be removed in order to provide access to the lateral cerebellomedullary cistern and allow a continuous egress of the CSF or irrigation fluid throughout the surgery.
 The important neural structures, such as the cochlear and facial nerves, should be identified early.
The important neural structures, such as the cochlear and facial nerves, should be identified early.
 The tumor should be initially debulked.
The tumor should be initially debulked.
 The dissection from the surrounding structures should be performed only after sufficient internal decompression is achieved.
The dissection from the surrounding structures should be performed only after sufficient internal decompression is achieved.
 The dissection should always be performed in the arachnoid plane.
The dissection should always be performed in the arachnoid plane.
 Bipolar coagulation, especially in the vicinity of a cranial nerve, should be avoided.
Bipolar coagulation, especially in the vicinity of a cranial nerve, should be avoided.
Lateral Approaches
The main anatomical obstacle for exposure of the CP angle and of the petroclival area laterally is the petrous bone. Multiple operative techniques, including partial or complete resection of the petrous bone—the so-called transpetrous approaches—have been developed in the past decades. They differ in the approach to the petrous bone—subtemporal or transmastoid, as well as in the amount of bone removal.21 The advantages of these lateral cranial base approaches include shorter distance to the tumor and surrounding neurovascular structures, improved visualization, and minimized brain retraction.22 A major drawback is their higher approach-related morbidity rate. Our experience and the evaluation of the long-term outcome, however, prove that simpler and safer approaches lead to better outcome and do not limit the possibility to remove the tumor completely.14,16,23,24 The complete visualization of both the tumor and of all adjacent structures is not a prerequisite for better outcome.
The middle fossa approach is frequently used in cases of small intrameatal VSs: the tumor is exposed via a temporal craniotomy and removal of the bone overlying the IAC. The adequate access to a larger tumor requires more bone removal, including the resection of the petrous apex behind the horizontal segment of the petrous internal carotid artery medial to the IAC and incision of the tentorium (the extended middle fossa approach and the Kawase approach).14,22
Although the terminology used to describe the transmastoid approaches is quite variable,4 the main categories, in respect to the amount of drilling of the pyramid, are the presigmoid approach, the retrolabyrinthine petrosectomy, the translabyrinthine petrosectomy, and the transcochlear approach or complete petrosectomy. The presigmoid approach combines a supra- and infratentorial exposure with various degrees of petrosectomy. The retrolabyrinthine approach implies removal of the bone between the sigmoid sinus and the semicircular canals and allows hearing preservation. If the semicircular canals and the lateral wall of the IAC are removed, a large exposure both of the intrameatal and extrameatal tumor portions is gained (translabyrinthine approach). The transcochlear approach or complete petrosectomy allows for better visualization of the structures anterior to the IAC and petroclival space. It is used in large tumors in patients with complete hearing loss.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree