Chapter 12 Cerebrovascular Occlusive Disease and Carotid Surgery
• Atherosclerotic occlusive disease is the most commonly seen cervical common carotid bifurcation and involves the common, internal carotid arteries (ICAs). Other causes of ischemia are intracranial atherosclerotic narrowing or occlusion; extracranial or intracranial dissection of the internal carotid, vertebral, and other arteries; and moyamoya disease.
• Ischemic disease becomes symptomatic owing to distal thromboembolism or diminished flow. Symptoms may include stroke and transient ischemic attacks.
• Workup of a patient presenting with ischemic stroke may include magnetic resonance imaging (MRI; diffusion-weighted images and MR perfusion), magnetic resonance angiography (MRA), computed tomography angiography (CTA), carotid duplex ultrasonography, transcranial Doppler angiography, and digital subtraction angiography (DSA).
• Patients with symptomatic cervical ICA stenosis greater than 50% benefit from carotid endarterectomy at high-volume centers with low complication rates.
• Endovascular carotid artery stenting (CAS) has been evaluated as an alternative to carotid endarterectomy in two randomized international trials. The short-term results indicate higher complication rates with CAS. It may be performed in selected patients, especially with very high carotid lesions.
• Moyamoya disease is caused by progressive occlusion of the intracranial ICA, and adjacent major branches, with accompanying dilation of small collateral arteries. It may manifest in children with transient ischemic attack or strokes and in adults with ischemic symptoms or hemorrhage. Prevention of further ischemic episodes may be accomplished by indirect revascularization (encephalodural/glial/myosynangiosis) or direct revascularization (extracranial bypass, most commonly superficial temporal artery to middle cerebral artery anastomosis).
• At present, randomized trials do not support the use of external carotid/internal carotid (EC-IC) arterial bypass for chronic atherosclerotic ischemic disease.
The central nervous system is metabolically demanding, receiving approximately 20% of cardiac output despite comprising only 2% of body weight. Cerebral blood flow (CBF) is directly proportional to the difference between mean arterial pressure (MAP) and intracranial pressure (ICP) and inversely related to cerebrovascular resistance (CVR) as per Ohm’s law. Alteration of cerebrovascular tone allows for maintenance of cerebral perfusion pressure over a wide range of mean arterial pressures. However, if cerebral perfusion pressure drops below 20 mm Hg, in the setting of arterial occlusion, for example, inadequate delivery of oxygen to brain tissue results in ischemia and subsequent infarction if CBF is not quickly returned to normal.1
The differential diagnosis for arterial occlusion includes both acute and chronic disease processes that may affect either intracranial or extracranial vessels. This chapter focuses on cerebrovascular occlusive disease processes commonly encountered and treated by the neurosurgeon—atherosclerotic cerebrovascular occlusive disease (with a specific emphasis on carotid artery stenosis), moyamoya disease, and cerebral arterial dissection. Following a brief review of clinical anatomy, the pathophysiology, clinical presentation, diagnosis, and management of each of the aforementioned conditions will be discussed. The final section of this chapter will detail the neurosurgical technique for carotid endarterectomy and superficial temporal artery to middle cerebral artery bypass, important surgical treatments for cerebrovascular occlusive disease in the armamentarium of the neurovascular surgeon.
Review of Clinical Anatomy
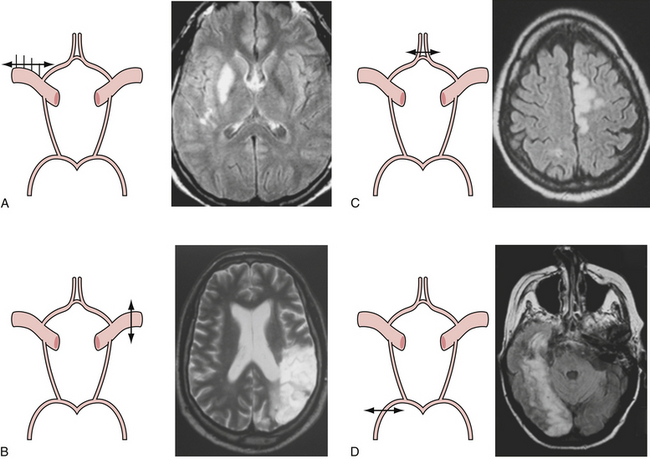
Presenting symptoms of acute occlusion reflect the respective vascular territories (Fig. 12.1). Anterior circulation involvement may manifest as monocular blindness and an absent pupillary light response; hemispheric signs such as contralateral homonymous hemianopia, hemiparesis, and hemisensory loss; specific signs of dominant hemispheric ischemia including aphasia, alexia, agraphia, acalculia, and dysarthria; and nondominant hemispheric symptoms including visuospatial neglect, constructional apraxia, loss of prosody of speech, and anosognosia. Posterior circulation symptoms, aside from alteration in level of consciousness, include motor deficits such as hemiparesis, tetraparesis, and facial paresis from brainstem lesions, vertigo, vomiting, pupillary abnormalities, ataxia, oculomotor signs, and pseudobulbar manifestations.
Atherosclerotic Cerebrovascular Occlusive Disease
Clinical Features
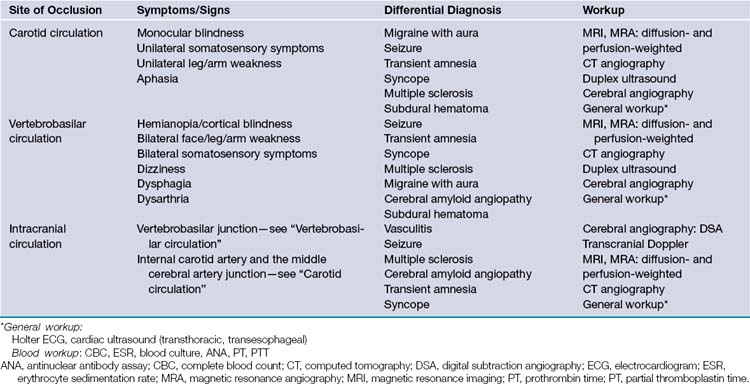
Given the chronic nature of cerebrovascular atherosclerosis, the cerebrovascular system can show a remarkable level of resilience prior to symptomatic presentation and often remains undiagnosed for many years. The remainder of the discussion will be focused on carotid disease given its responsiveness to neurosurgical intervention. Vertebrobasilar and intracranial atherosclerosis share a similar clinical presentation with carotid artery stenosis but specific neurological findings are localized to the vascular territories involved (Table 12.1).
Diagnosis
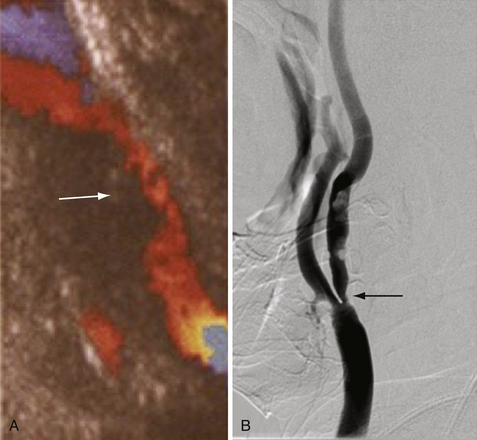
The radiological evaluation of cerebrovascular atherosclerotic disease, specifically disease of the carotid arteries, consists of identifying the level and location of stenosis/occlusion, defining the etiology of these lesions, surgical planning, and patient follow-up. The four major modalities (most invasive to the least invasive techniques) are digital subtraction cerebral angiography (CA), computed tomographic angiography (CTA), magnetic resonance angiography (MRA), and duplex ultrasound (DUS).2–4
Cerebral angiography is the gold standard for imaging the carotid arteries (Fig. 12.2). Cerebral angiography has superior accuracy compared to noninvasive techniques, which may overestimate or underestimate the degree of stenosis, an important characteristic for accurately determining the extent of disease and for surgical planning. Moreover, more than one noninvasive modality is usually required to perform an accurate and comprehensive assessment of atherosclerotic disease. The advent of digital subtraction angiography (DSA) has reduced the size of catheter needed, the amount of contrast required, and the duration of this procedure. Although there is lower spatial resolution, DSA allows for dynamic visualization of blood flow at the site of stenosis as well as collateralization and flow around the vascular lesion; this information provides an indication of the clinical impact of the stenosis. Patients should be screened for history of adverse reaction to contrast agent and renal disease, as contrast nephropathy and allergy are potential complications of cerebral angiography.
CTA combines CT technique with venous injection of contrast dye to visualize the supra-aortic vessels (both intracranially and extracranially). Unlike CA and DSA, CTA provides an anatomical description of the surrounding structures in addition to the vasculature, which is extremely useful in identifying nonatherosclerotic causes of stenosis. This technique is less invasive than DSA but requires contrast bolus comparable to angiography, and so contrast allergy and nephropathy are possible complications. Furthermore, as the quality and accuracy of the obtained image depends on both the timing of the injection and the scan itself, CTA often suffers from overestimation or underestimation of the degree of disease.
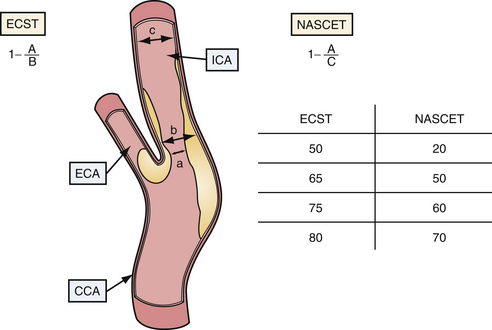
Measurement of Carotid Artery Stenosis
Current indications for surgical intervention of carotid atherosclerotic disease require objective and reproducible methods to evaluate the degree of stenosis. Two major methods of measuring carotid stenosis were developed for use in the major clinical trials evaluating the efficacy of carotid endarterectomy: the North American Symptomatic Carotid Endarterectomy Trial (NASCET)5 method and the European Carotid Surgery Trial (ESCT)6 method. The primary difference in these methods lies in how the observer estimates the diameter of the reference vessel. The NASCET utilizes the normal carotid wall just distal to the stenotic lesion as the reference vessel, and ESCT defines the reference as the estimated diameter of the carotid bulb. Figure 12.3 diagrams each method of measurement and the mathematical relationship between these two methods. Note that the ECST and NASCET approximations are comparable with severe disease, but that their values diverge when the stenosis is not as pronounced. In contemporary practice, most patients are determined to have high-grade (>60-70%) stenosis on the basis of noninvasive color flow Doppler, CTA, or MRA. When two of these three modalities agree on the degree of stenosis and no other modality questions the result, the correlation with catheter angiography is excellent. Given the risk of catheter angiography, this is generally reserved for patients in whom the studies are not concordant.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree