The Limbic Lobes and the Neurology of Emotion: Introduction
The medical literature is replete with references to illnesses based on emotional disorders. Careful examination of clinical material discloses that diverse phenomena are being so classified: anxiety states, cycles of depression and mania, reactions to distressing life situations, psychosomatic diseases, and illnesses of obscure nature. Obviously, great license is being taken with the term emotional. Such ambiguity renders neurologic analysis difficult. Nevertheless, in certain clinical states patients appear to be excessively apathetic or elated under conditions that are not normally conducive to such displays of emotion. It is to these disturbances that the following remarks pertain. Emotion may be defined as any feeling state—for example, fear, anger, excitement, love, or hate—associated with certain types of bodily changes (mainly visceral and under control of the autonomic nervous system) and leading usually to an impulse to action or to a certain type of behavior. If the emotion is intense, there may ensue a disturbance of intellectual functions, that is, a disorganization of rational thought and a tendency toward a more automatic behavior of unmodulated, stereotyped character.
In its most easily recognized human form, emotion is initiated by a stimulus, real or imagined, the perception of which involves recognition, memory, and specific associations. The emotional state that is engendered is mirrored in a psychic experience, i.e., a feeling, which is purely subjective and known to others only through the patient’s verbal expressions or by judging his behavioral reactions. This behavioral aspect, which is in part autonomous (hormonal–visceral) and in part somatic, shows itself in the patient’s facial expression, bodily attitude, vocalizations, or directed voluntary activity, an observable display for which we use the term affect. In other words, the components of emotion appear to consist of (1) the perception of a stimulus, which may be internal (an idea) or external, (2) the feeling, (3) the autonomic–visceral changes, (4) the outward display (affect), and (5) the impulse to a certain type of activity. In many cases of neurologic disease, it is not possible to separate these components from one another, and to emphasize one of them does no more than indicate the particular bias of the examiner. Obviously, neural networks of both affective response and cognition are involved.
The occurrence of abnormal emotional reactions in the course of disease is associated with lesions that preferentially involve certain parts of the nervous system. These structures are grouped under the term limbic and are among the most complex and least understood parts of the nervous system. The Latin word limbus means “border” or “margin.” Credit for introducing the term limbic to neurology is usually given to Broca, who used it to describe the ring of gray matter formed primarily by the cingulate and parahippocampal gyri that encircles the corpus callosum and underlying upper brainstem. Actually, Thomas Willis had pictured this region of the brain and referred to it as the limbus in 1664. Broca preferred his term, le grand lobe limbique, to rhinencephalon, which was the term then in vogue and referred more specifically to structures having an olfactory function. Neuroanatomists have extended the boundaries of the limbic lobe to include not only the cingulate and parahippocampal gyri but also the underlying hippocampal formation, the subcallosal gyrus, and the paraolfactory area. The terms visceral brain and limbic system, introduced by MacLean, have an even wider designation and more completely describe the structures involved in emotion and its expression; in addition to all parts of the limbic lobe, they include a number of associated subcortical nuclei such as those of the amygdaloid complex, septal region, preoptic area, hypothalamus, anterior thalamus, habenula, and central midbrain tegmentum, including the raphe nuclei and interpeduncular nucleus. The major structures that constitute the limbic system and their relationships are illustrated in Figs. 25-1 and 25-2.
Figure 25-1.
Sagittal diagram of the limbic system. A. Surface topography of the limbic system and associated prefrontal cortex. B. Connections of the limbic structures and their relation to the thalamus, hypothalamus, and midbrain tegmentum. The cortical parts of the limbic system, or limbic lobe, are interconnected by a septohypothalamic–mesencephalic bundle ending in the hippocampus, and the fornix, which runs from the hippocampus back to the mammillary bodies, and by tracts from the mammillary bodies to the thalamus and from the thalamus to the cingulate gyrus. The Papez circuit is the internal component of this system. See also Fig. 25-2 and the text. (Reproduced with permission from Kandel ER, Schwartz JH, Jessell TM: Principles of Neural Science, 4th ed. New York, McGraw-Hill, 2000.)
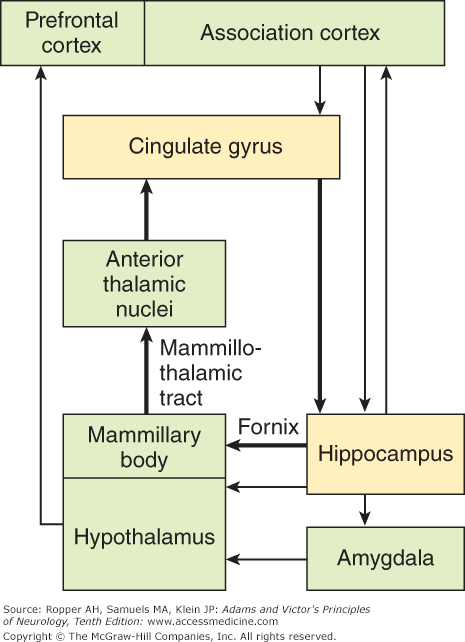
Figure 25-2.
Schematic block diagram of the limbic connections. The internal connections (bold lines) represent the circuit described by Papez. The external connections (thin lines) are more recently described pathways. This figure also shows the connections to the amygdala and prefrontal and association cortices. (Reproduced with permission from Kandel ER, Schwartz JH, Jessell TM: Principles of Neural Science, 4th ed. New York, McGraw-Hill, 2000.)
The cytoarchitectonic arrangements of the limbic cortex clearly distinguish it from the surrounding neocortex. The latter, as stated in Chap. 22, differentiates into a characteristic six-layer structure (isocortex). In contrast, the inner part of the limbic cortex, the hippocampus, is composed of irregularly arranged aggregates of nerve cells that tend to be in a trilaminate configuration (archi- or allocortex). The cortex of the cingulate gyrus, which forms the outer ring of the limbic lobe, is transitional between neocortex and allocortex—hence, it is called mesocortex. The entorhinal cortex adjacent to the anterior hippocampus has a similar transitional architecture. Information from a wide array of cortical neurons is funneled into the dentate gyrus and then to the CA (cornu ammonis) pyramidal cells of the hippocampus. Output from the hippocampus is mainly from the pyramidal cells of the CA1 segment and subiculum, whose axons form the fibria and fornix. The amygdaloid complex, a subcortical nuclear component of the limbic system, also has a unique composition, consisting of several separable nuclei, each with connections to other limbic structures.
The connections between the orbitofrontal neocortex and limbic lobes, between the individual components of the limbic lobes, and between the limbic lobes and the hypothalamus and midbrain reflect their many functional relationships in regard to emotion. At the core of this system lies the medial forebrain bundle, a complex set of ascending and descending fibers that connect the orbitomesiofrontal cortex, septal nuclei, amygdala, and hippocampus rostrally, and certain nuclei in the midbrain and pons caudally. This system, of which the hypothalamus is the central part, was designated by Nauta as the septohypothalamo–mesencephalic continuum.
There are many other interrelationships among various parts of the limbic system, only a few of which can be indicated here. The best known of these is Papez circuit. It leads from the hippocampus, via the fornix, to the mammillary body and septal and preoptic regions (see Fig. 25-1). The mammillothalamic tract (bundle of Vicq d’Azyr) connects the mammillary nuclei with the anterior nuclei of the thalamus, which, in turn project to the cingulate gyrus and then, via the cingulum back to the hippocampus. The cingulum runs concentric to the curvature of the corpus callosum; it connects various parts of the limbic lobe to one another and projects to the striatum and to certain brainstem nuclei as well. Also, the cingulum receives fibers from the inferior parietal lobule and temporal lobe, which are multimodal association centers for the integration of visual, auditory, and tactile perceptions. It is connected to the opposite cingulum through the anterior corpus callosum.
The functional properties of the limbic structures first became known during the third and fourth decades of the twentieth century. From ablation and stimulation studies, Cannon, Bard, and others established the fact that the hypothalamus contains the suprasegmental integrations of the autonomic nervous system, both the sympathetic and parasympathetic parts. Soon after, anatomists found efferent pathways from the hypothalamus to the neural structures subserving parasympathetic and sympathetic reflexes. One such segmental reflex, involving the sympathetic innervation of the adrenal gland, served as the basis of Cannon’s emergency theory of sympathoadrenal action, which for many years dominated thinking about the neurophysiology of acute emotion.
Following Cannon, Bard incorrectly localized the central regulatory apparatus for respiration, wakefulness, and sexual activity in the hypothalamus. Only later, the hypothalamus was found to contain neurosecretory cells, which control the secretion of the pituitary hormones; also within it are special sensory receptors for the regulation of hunger, thirst, body temperature, and levels of circulating electrolytes. Gradually the idea emerged of a hypothalamic–pituitary–autonomic system that is essential to both the basic homeostatic and emergency (“fight-or-flight”) reactions of the organism. The functional anatomy of these autonomic and neuroendocrine systems is discussed in Chaps. 26 and 27.
The impression of the leading psychologists of the nineteenth century that autonomic reactions were the essential motor component of instinctual feeling has been partially corroborated. It was proposed that emotional experience was merely the self-awareness of these visceral activities (the James-Lange theory of emotion alluded to in Chap. 24). The limitations of this theory became evident when it was demonstrated by Cannon that the capacity to manifest emotional changes remained after all visceral afferent fibers had been interrupted. Nonetheless, it remains true that perception of visceral activities can greatly alter the emotional state. An example is the perception of a rapid heartbeat, leading to heightened anxiety, which results in further acceleration in the heart rate.
Although the natural stimuli for emotion involve the same neocortical perceptive–cognitive mechanisms, as do nonemotional sensory experiences, there are important differences, which relate to the prominent visceral effects and particular behavioral reactions evoked by emotion. Clearly, specific parts of the nervous system must be utilized. Bard, in 1928, first produced “sham rage” in cats by removing the cerebral hemispheres and leaving the hypothalamus and brainstem intact. This is a state in which the animal reacts to all stimuli with expressions of intense anger and signs of autonomic overactivity. In subsequent studies, Bard and Mountcastle found that only if the ablations included the amygdala on both sides would sham rage be produced; removal of all the neocortex, but sparing of the limbic structures resulted in placidity. Interestingly, in the macaque, a normally aggressive and recalcitrant animal, removal of the amygdaloid nuclei bilaterally greatly reduced the reactions of fear and anger (see further on). The role of the hypothalamus and amygdala in the production of both directed and undirected anger and displays of rage has turned out to be far more complex. In any case, Papez, on the basis of these and his own anatomic observations, postulated that the limbic parts of the brain elaborate the functions of central emotion and participate as well in emotional expression. The intermediate position of the limbic structures enables them to transmit neocortical effects from their outer side to the hypothalamus and midbrain on their inner side.
The role of the cingulate gyrus in the behavior of animals and humans has been the subject of much discussion. Stimulation is said to produce autonomic effects similar to the vegetative correlates of emotion (increase in heart rate and blood pressure, dilatation of pupils, piloerection, respiratory arrest, breathholding). More complex responses, such as fear, anxiety, or pleasure, have been reported during neurosurgical stimulative and ablative procedures, although these results are inconsistent. Bilateral cingulectomies performed in the past on psychotic and anxious patients result in an overall diminution of emotional reactions (Ballantine et al; Brown). Some investigators believe that the cingulate gyri are also involved in memory processing (functioning presumably in connection with the mediodorsal thalamic nuclei and mediotemporal lobes) and in exploratory behavior and visually focused attention. In humans, this system appears to be more efficient in the nondominant hemisphere. According to Bear, and as conceptualized by Baleydier and Mauguiere, the cingulate gyri serve dual functions in cognition and in emotional reactions.
Another aspect of limbic function has come to light with information about the neurotransmitters that interconnect the structures within the system. The concentration of norepinephrine is highest in the hypothalamus and next highest in the medial parts of the limbic system; at least 70 percent of this monoamine is concentrated in terminals of axons that arise in the medulla and in the locus ceruleus of the rostral pons. Axons of other ascending fibers, especially those originating in the reticular formation of the midbrain and terminating in the amygdala and septal nuclei as well as in lateral parts of the limbic lobe are rich in serotonin.
The axons of neurons in the ventral tegmental parts of the midbrain, which ascend in the medial forebrain bundle and the nigrostriatal pathway, contain a high content of dopamine. Perhaps this explains the observation that a severe depressive reaction may be produced by electrical stimulation of the substantia nigra with an aberrantly placed electrode for the treatment of Parkinson disease (see Chap. 39). The many structures listed above and their connections certainly constitute a unified functional system. The term limbic system is a simplification, particularly as the various parts differ widely in respect to their connections with the neocortex and central nuclei, their transmitters, and their effects when damaged. But it can be said that lesions in this system most consistently and specifically alter emotionality; thus it remains a useful concept.
Emotional Disturbances Due to Diseases Involving Limbic Structures
Many of the foregoing ideas about the role of the limbic system have come from experimentation in laboratory animals. Only in relatively recent years have neurologists, primed with the knowledge of these studies, begun to relate emotional disturbances in patients with disease of limbic structures. These clinical observations, summarized in the following pages, form an interesting chapter in neurology. Table 25-1 lists the most readily recognized disturbances of emotion. The list is tentative, as our understanding of many of these states, particularly their pathologic basis, is incomplete. Only a small number of these derangements can be used as indicators of lesions and diseases in particular parts of the human brain. Taken in context, however, these disturbances are useful diagnostically. As knowledge of emotional disorders increases, an understanding of the functioning of limbic structures will undoubtedly bring together large segments of psychiatry and neurology.
|
These are portrayed by the patient with a florid delirium. Threatened by imaginary figures and voices that seem real and inescapable, the patient trembles, struggles to escape, and displays the full picture of terror. The patient’s affect, emotional reaction, and visceral and somatic motor responses are altogether appropriate to the content of hallucinations. We have seen a patient slash his wrists and another try to drown himself in response to hallucinatory voices that admonished them for their worthlessness and the shame they had brought on their families. But the abnormality in these circumstances is one of disordered perception and thinking, and we have no reason to believe that there is a fundamental derangement of the mechanisms for emotional expression.
There also occurs a state, difficult to classify, of overwhelming emotionality in patients who are in severe, acute pain. The patient’s attention can be captured only briefly, but within moments, there is a return to an extreme state of angst, groaning, and anger. We have encountered this with spinal subdural hemorrhage, subarachnoid hemorrhage, explosive migraine, trauma with multiple fractures, and intense pelvic, renal, or abdominal pain, all understandable as responses to extralimbic stimuli.
It is a commonplace clinical experience that cerebral diseases of many types, seemingly without respect to location, weaken the mechanism of control of emotional expression. A patient whose cerebrum has been damaged, for example, by a series of vascular lesions, may suffer the humiliation of crying in public upon meeting an old friend or hearing the national anthem, or of displaying uncontrollable laughter in response to a mildly amusing remark or an attempt to tell a funny story. There may also be easy vacillation from one state to another, an emotional lability that has for more than a century been accepted as a sign of “organic brain disease.” In this type of emotional disturbance, the response, while excessive, does not quite reach the degree of forced emotionality of the special form of lability described as pseudobulbar (see below); furthermore, it is appropriate to the stimulus and the affect is congruent with the visceral and motor components of the expression. The anatomic substrate is obscure. Perhaps lesions of the frontal lobes more than of other parts of the brain are conducive to this state, but the authors are unaware of a critical clinicoanatomic study that substantiates this impression. Emotional lability is a frequent accompaniment of diffuse cerebral diseases, such as Alzheimer disease, but these diseases involve the limbic cortex as well. Also under this heading might be included the tearfulness and facile mood that so often accompany chronic diseases of the nervous system, and the shallow facetiousness (witzelsucht) and behavioral disinhibition of the patient with frontal lobe disease.
This form of disordered emotional expression, characterized by outbursts of involuntary, uncontrollable, and stereotyped laughing or crying, has been recognized since the late nineteenth century. Numerous references to these conditions (the Zwangslachen and Zwangsweinen noted by German neurologists and the rire et pleurer spasmodiques described by the French) can be found in the writings of Oppenheim, von Monakow, and Wilson (see Wilson for historical references). The term emotional incontinence applied by psychiatrists may be accurate but is a bit pejorative. Forced laughing or crying always has a pathologic basis in the brain, either diffuse or focal; hence, this stands as a syndrome of multiple causes. It may occur with degenerative and vascular diseases of the brain (Table 25-2) and no doubt is the direct result of them, but often the diffuse nature of the underlying disease precludes useful topographic analysis and clinicoanatomic correlation.
Bilateral strokes (lacunes in the cerebral hemispheres or pons) most often after several strokes in succession |
Binswanger diffuse leukoencephalopathy (Chap. 34) |
Amyotrophic lateral sclerosis with pseudobulbar palsy |
Progressive supranuclear palsy |
Multiple sclerosis with bilateral corticobulbar demyelinative lesions |
Bilateral traumatic lesions of the hemispheres |
Gliomatosis cerebri |
Hypoxic–ischemic encephalopathy |
Pontine myelinolysis |
Wilson disease |
The best examples of pathologic laughing and crying are provided by multiple lacunar vascular disease and by amyotrophic lateral sclerosis, multiple sclerosis, and progressive supranuclear palsy, in each case the lesions being distributed bilaterally and generally involving the motor tracts, specifically, the corticobulbar motor system as discussed further on. They may also be part of the residue of the more widespread lesions of hypoxic–ischemic encephalopathy, Binswanger ischemic encephalopathy, cerebral trauma, infiltrative gliomas of the frontal lobe or pons, and infectious and noninfectious encephalitides. Typical in our experience is a sudden hemiplegia from a stroke that is engrafted upon a preexistent (and often clinically silent) lesion in the opposite hemisphere; this sets the stage for the pathologic displays of emotionality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree