The Hypothalamus and Neuroendocrine Disorders: Introduction
The hypothalamus plays three roles in the actions of the nervous system. The first, as the “head ganglion” of the autonomic nervous system, was described in the preceding chapter; the second, as the circadian and seasonal clock for behavioral and sleep–wake functions, was considered in Chap. 19 on sleep; and the third, as the neural center of the endocrine system, is the subject of this chapter. In the hypothalamus, these systems are integrated with one another as well as with neocortical, limbic, and spinal influences. Together, they maintain homeostasis and participate in the substructure of emotion and affective behavior.
The expansion of knowledge of neuroendocrinology during the past few decades stands as one of the significant achievements in neurobiology. It has been learned that neurons, in addition to transmitting electrical impulses, can synthesize and secrete complex molecules locally and into the systemic circulation, and that these molecules are capable of activating or inhibiting endocrine, renal, and vascular cells at distant sites.
The concept of neurosecretion probably had its origins in the observations of Speidel, in 1919, who noted that some of the hypothalamic neurons had the morphologic characteristics of glandular cells. Their suggestion that such cells might secrete hormones into the bloodstream was so novel, however, that it was rejected by most biologists at the time. This seems surprising now that neurosecretion is viewed as a fundamental part of the science of endocrinology.
Following these early observations, it was found that peptides secreted by neurons in the central and peripheral nervous systems were also contained in glandular cells of the pancreas, intestines, and heart. This seminal observation was made in 1931 by Euler and Gaddum, who isolated a substance from the intestines that was capable of acting on smooth muscle and called it “P” (from powder). But it was not until some 35 years later that Leeman and her associates purified an 11-amnio-acid peptide that is now called substance P (see Aronin et al). Then followed the discovery of six hypothalamic mediators of anterior pituitary hormone secretion: TRH, and somatostatin 1973, GnRH, CRH, and GHRH. In the background was always dopamine that acted as an inhibitor of pituitary hormone secretion. Subsequently, a number of other neuropeptides including enkephalin, neuropeptide Y, orexin as discussed in Chap. 19.
The Hypothalamus
The hypothalamus lies on each side of the third ventricle and is continuous across the floor of the ventricle. It is bounded posteriorly by the mammillary bodies, anteriorly by the optic chiasm and lamina terminalis, superiorly by the hypothalamic sulci, laterally by the optic tracts, and inferiorly by the hypophysis. It comprises three main nuclear groups: (1) the anterior group, which includes the preoptic, supraoptic, and paraventricular nuclei; (2) the middle group, which includes the tuberal, arcuate, ventromedial, and dorsomedial nuclei; and (3) the posterior group, comprising the mammillary and posterior hypothalamic nuclei.
Nauta and Haymaker have subdivided the hypothalamus sagittally. The lateral part lies lateral to the fornix; it is sparsely cellular and its cell groups are traversed by the medial forebrain bundle—which carries finely myelinated and unmyelinated ascending and descending fibers to and from the rostrally placed septal nuclei, substantia innominata, nucleus accumbens, amygdala, and piriform cortex—and the caudally placed tegmental reticular formation. The medial hypothalamus is rich in cells, some of which are the neurosecretory cells for pituitary regulation and visceral control. It contains two main efferent fiber systems—the mammillothalamic tract of Vicq d’Azyr (named for the physician to Louis XIV, a paramour of Marie Antoinette), which connects the mammillary nuclei with the anterior thalamic nucleus (which, in turn, projects to the cingulate gyrus), and the mammillotegmental tract. Additional structures of importance are the stria terminalis, which runs from the amygdala to the ventromedial hypothalamic nucleus, and the fornix, which connects the hippocampus to the mammillary body, septal nuclei, and periventricular parts of the hypothalamus. The lateral and medial parts of the hypothalamus are interconnected and their functions are integrated.
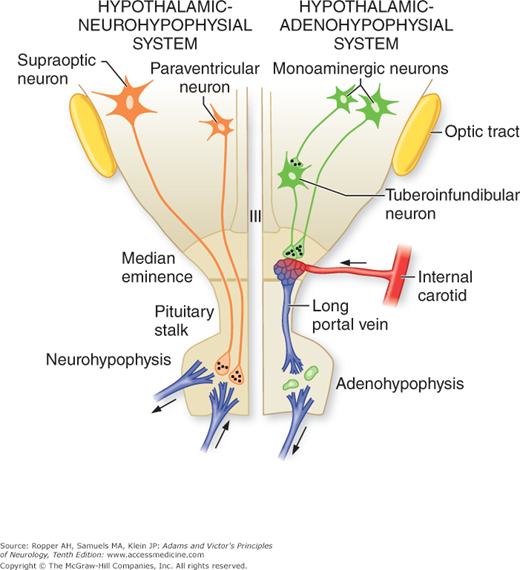
The inferior surface of the hypothalamus bulges downward from the floor of the fourth ventricle; this region is known as the tuber cinereum. The infundibulum is a critical structure that arises from the tuber cinereum. The superior hypophyseal artery, derived from the internal carotid artery, forms a network of capillaries in the median eminence, the veins of which are the critical portal system (Fig. 27-1).
Figure 27-1.
Diagram of the hypothalamic–pituitary axis. Indicated on the left is the hypothalamic–neurohypophyseal system, consisting of supraoptic and paraventricular neurons, axons of which terminate on blood vessels in the posterior pituitary (neurohypophysis). The hypothalamic–adenohypophyseal system is illustrated on the right. Tuberoinfundibular neurons, the source of the hypothalamic regulatory hormones, terminate on the capillary plexus in the median eminence. (Courtesy of Dr. J.B. Martin.)
The median eminence assumes special importance because of the intimate relation of its cells to the vessels of the portal system that bathe the anterior lobe of the pituitary gland. The releasing hormones of the hypothalamus are delivered directly to their target cells in the anterior pituitary via the portal vessels extending through the stalk, thereby avoiding systemic dilution of these factors. In this way the portal system represents the interface between converging pathways from the brain and the pituitary. The tuberoinfundibular neurons of the arcuate nucleus and anterior periventricular nuclei synthesize most of the releasing factors described in Fig. 27-1.
The infundibulum extends into the pituitary stalk, which in turn, enters the neurohypophysis. The infundibulum is a structure that stands out because it contains the median eminence as well as the neurohypophyseal fibers, containing vasopressin and oxytocin, which course through the infundibulum on their way to the posterior pituitary. The main blood supply to the posterior pituitary is from the inferior hypophyseal artery that is a branch of the cavernous part of the internal carotid artery.
The abundant blood supply of the hypothalamus (from several feeding arteries) is of importance to neurosurgeons who attempt to obliterate aneurysms that derive from adjacent vessels. Many small radicles, arising, not just from the carotid arteries, but also the posterior and anterior communicating arteries as well as from the most proximal portions of the anterior and posterior cerebral arteries, form a network of such redundancy that infarction of the hypothalamus is infrequent. The venous drainage from the portal system is to the petrosal sinus, where hormone levels can be sampled.
Readers requiring a more extensive source of information on anatomic and other aspects of the hypothalamus are directed to the comprehensive material by Swaab in the two-volumes of the Handbook of Clinical Neurology devoted to this subject, and to the monograph by Martin and Reichlin.
HORMONE | TARGET GLAND | SECRETORY CELL DESIGNATION | AMINO ACIDS | NORMAL RANGE |
|---|---|---|---|---|
Growth hormone (GH) | Liver, other organs | Somatotrope | 191 | <0.5 μg/L per 24 h |
ACTHa | Adrenal | Coritcotrope | 39 | 4–22 pg/L |
Prolactin (PRL) | Breast, other tissues | Lactotrope | 199 | M <15 μg/L; F <20 μg/L |
Thyroid-stimulating hormone (TSH) | Thyroid | Thyrotrope | 211 | 0.1–0.5 mU/L |
FSH and LHb | Ovary, testes | Gonadotrope | 210; 201 | M 5–20 IU/L; F 2–20 IU/L (basal level) |
This was the first of the releasing hormones to be identified; its tripeptide structure was determined in 1968. The hormone is elaborated by the anterior periventricular, paraventricular, arcuate, ventromedial, and dorsomedial neurons, but not by those of the posterior hypothalamic or thalamic nuclei. It stimulates the release of thyroid-stimulating hormone (TSH) from the pituitary gland. TSH, in turn, increases the activity of every step of the synthesis of thyroid hormone and stimulates the release of T4 (thyroxine) and T3 (triiodothyronine). Pituitary cells that release dopamine and somatostatin are also stimulated to a slight degree; the latter has an inhibitory effect on TSH. There is also an inhibitory feedback of T3 on TSH and TRH. Actually, more than half of brain TRH is found outside the hypothalamus—in brainstem raphe nuclei, tractus solitarius, and the anterior and lateral horn cells of spinal cord—suggesting that TRH may function as a central regulator of the autonomic nervous system.
This hormone and somatostatin (also known as growth hormone release-inhibiting hormone, or SRIH) are both secreted by specialized tuberoinfundibular neurons and released into the hypophyseal–portal circulation, by which they are carried to specific growth hormone (GH) secreting cells of the anterior pituitary gland (somatotropes). Immunohistochemical staining has shown the sources of GHRH and somatostatin to be neurons of the posterior part of the arcuate and ventromedian hypothalamic nuclei and other neurons of the median eminence and premammillary area. Somatostatin, a 14-amino-acid peptide, is produced more anteriorly by neurons in the periventricular area and small cell part of the paraventricular nucleus. The amygdala, hippocampus, and other limbic structures project to the arcuate nuclei via the medial corticohypothalamic tract (in the stria terminalis) and are believed to account for the sleep- and stress-induced fluctuations of GH and somatostatin. Also, it has been demonstrated that all 4 biogenic amines (dopamine, norepinephrine, epinephrine, and serotonin) influence GH regulation, as does acetylcholine, either by direct action on pituitary somatotropic cells or on hypothalamic regulatory neurons. TRH also increases GH from somatotropes. Many of the latter pituitary cells contain large eosinophilic granules, but others, previously identified incorrectly as chromophobe cells, also contain GH. Somatomedin C, a basic peptide that is synthesized in the liver, exerts feedback control of GH by inhibiting the pituitary somatotropes and stimulating the release of somatostatin. Growth hormone enhances skeletal growth by stimulating the proliferation of cartilage and growth of muscle. It also regulates lipolysis, stimulates the uptake of amino acids in cells, and has anti-insulin effects. The blood concentrations of GH fluctuate from 1 or 2 ng/mL to more than 60 ng/mL, being highest within the first hour or two after the onset of sleep.
This hormone, a 14-amino-acid peptide, acts synergistically with vasopressin to release adrenocorticotropic hormone (ACTH) from basophilic cells in the pituitary. ACTH stimulates the synthesis and release of the hormones of the adrenal cortex, mainly glucocorticoids (cortisol or hydrocortisone) but also mineralocorticoids (aldosterone) and androcorticoids (converted in the tissues to testosterone). The neurons of origin of CRH lie in a particular part of the paraventricular nucleus, other cells of which form the paraventricular–supraopticohypophysial tract (neurohypophysis) and elaborate vasopressin, oxytocin, and several other substances (neurotensin, dynorphin, vasoactive intestinal peptide). These hypothalamic cells receive an extensive input from other regions of the nervous system, particularly via the noradrenergic pathways (from reticular neurons in the medulla and those of the locus ceruleus and tractus solitarius) and from many of the limbic structures. Presumably, these extrahypothalamic connections provide the mechanism by which stress and pain activate the secretion of ACTH and cortisol. CRH itself is widely distributed in the brain. There is feedback control of CRH and ACTH via glucocorticoid receptors in the hypothalamus and anterior lobe of the pituitary. Serotonin and acetylcholine enhance ACTH secretion, whereas the catecholamines are inhibitory.
This 10-amino-acid peptide originates in the arcuate nucleus and is present in highest concentration near the median eminence. It effects the release of the two gonadotropic hormones—luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The ovary and testis, by secreting a peptide called inhibin, are able to suppress FSH, as do gonadal steroids—i.e., estrogens. GnRH is under the influence of other neuronal systems, which are modulated by catecholamines, serotonin, acetylcholine, and dopamine. Puberty, menstruation, ovulation, lactation, and menopause are all related to the effects of GnRH, FSH, and LH on the ovaries, uterus, breasts, and testes. Normal levels of blood FSH are 2.5 to 4.9 ng/mL in prepuberty and 7.5 to 11 ng/mL in the adult; levels of blood LH are 2.8 to 9.6 ng/mL in prepuberty and 10 to 18 ng/mL in the adult.
Dopamine is released by neurons in the region of the arcuate nucleus and inhibits lactotrophic cells of the anterior pituitary. The hypothalamopituitary axis is responsive to sensory stimuli from the nipples, via pathways in the spinal cord and brainstem, accounting for the effect of suckling on milk production. Nipple stimulation is also an important influence on oxytocin secretion, as described later. The normal blood levels of prolactin are 5 to 25 ng/mL.
This inhibitory action of dopamine on prolactin secretion accounts for galactorrhea and reproductive disorders, which occur with tumors that compress the pituitary stalk. These interrupt the venous portal transport of dopamine from the hypothalamus to the dopamine-sensitive lactotrophs in the pituitary. This mechanism also explains galactorrhea following the administration of dopamine-blocking medications such as Haldol.
The oligopeptides vasopressin and oxytocin are elaborated by cells of the supraoptic and paraventricular nuclei and are transported, via their axons, through the stalk of the pituitary to its posterior lobe, where these substances are stored. Together, these elements constitute the neurohypophysis (posterior pituitary), which develops as an evagination of the floor of the third ventricle. Some of the vasopressin-containing nerve endings also terminate on cells of origin of the autonomic nervous system and on the capillary plexus of the hypophyseal portal circulation, through which they influence the secretion of CRH and GH. The delivery to the posterior pituitary of oxytocin and vasopressin is therefore through the nerve endings that originate in the hypothalamus and terminate in the neurohypophysis. There is some influence of anterior pituitary hormones. The peptide parts of vasopressin and oxytocin, whose chemical nature was determined by DuVigneaud, are almost identical, differing from one another by only two amino acids.
Vasopressin, acting on the V2 receptors in kidney tubules, serves as the antidiuretic hormone (ADH) and, complemented by thirst mechanisms, maintains the osmolality of the blood. Plasma osmolality modifies vasopressin secretion by acting directly on the supraoptic and paraventricular neurons or on separate osmoreceptors in the hypothalamus. The sensitivity of the vasopressin–ADH mechanism is demonstrated by the absence of antidiuretic effect when vasopressin is below 1 pg/mL and by maximal antidiuresis when plasma levels reach 5 pg/mL. If serum osmolality falls below 280 mOsm/L, the release of ADH is completely inhibited. This system is most effective in maintaining homeostasis when serum osmolality is relatively close to the normal range, between 280 and 295 mOsm/L.
Alterations in blood volume and pressure also affect vasopressin release through neural mechanisms that have their origin in baro- and mechanoreceptors of the aortic arch, carotid sinus, and right atrium. Afferent signals from these regions are conveyed in the vagus and glossopharyngeal nerves, which synapse in the nucleus of the tractus solitarius; the precise pathways to the hypothalamus have not been delineated, however. With severe hypotension, ADH release will continue even if there is a low serum osmolality, i.e., pressure predominates over osmolarity as a stimulus. Vasopressin secretion is also influenced by nonosmotic factors. Nausea, for example, is a potent stimulus, raising levels of the hormone 100-fold. Hypoglycemia has a less-profound effect. Drugs such as morphine, nicotine, alcohol, and certain chemotherapeutic agents (cyclophosphamide) also cause release of the stored peptide. Pain, emotional stress, and exercise have long been thought to cause release of vasopressin, but it is unclear whether this is a direct effect or is mediated through hypotension or nausea.
Oxytocin initiates uterine contraction and has milk-ejecting effects. Its release is stimulated by distention of the cervix, labor, breastfeeding, and estrogen. The effects of oxytocin are inhibited by alcohol.
In summary, it is apparent that the regulatory system of hypothalamic-releasing hormones is complex. The releasing factors have overlapping functions, and the hypothalamic nuclei act on many parts of the brain in addition to the pituitary. Conversely, many parts of the brain influence the hypothalamus through neural connections or modulate its activity and that of the pituitary gland through the action of neurotransmitters and modulators (catecholamines, acetylcholine, serotonin, and dopamine). There is feedback control between every part of the hypothalamus and the endocrine structures on which it acts. The factors that influence hypothalamic neurons have been reviewed in detail by Reichlin. Some of these relationships have been mentioned and others will emerge further on in this chapter and in later chapters, particularly as they relate to behavioral and psychiatric disorders.
Of particular significance is the role of the hypothalamus in the integration of the endocrine and autonomic nervous systems at both the peripheral and central levels. The best-known example of this interaction is in the adrenal medulla, as indicated in Chap. 26. Similarly, the juxtaglomerular apparatus of the kidney and the islets of Langerhans of the pancreas function as neuroendocrine transducers, in that they convert a neural stimulus (in these cases adrenergic) to an endocrine secretion (renin from the kidney and glucagon and insulin from the islet cells).
The hypothalamus also plays a critical role in the development of human sexuality and its expression, a theme elaborated in the next chapter. The suprachiasmatic nucleus and the number of neurons it contains are considerably larger in men than in women, a dimorphism that becomes evident during postnatal development. The interstitial nucleus of the hypothalamus is reportedly smaller in the homosexual male, evidence perhaps that homosexuality has a recognizable morphologic basis (Levay). This biologic evidence has been sharply challenged (Byne). These issues are addressed further in the section on sexual development in Chap. 28. The intimate relationship of the hypothalamus with the development of sexual characteristics at all stages of life is shown by the appearance, in the infundibular area, of hypertrophic neurons that are rich in estrogen receptors; it has been proposed that some of the symptoms of menarche are timed and mediated by these hypothalamic neurons. With aging, and more so in Alzheimer disease, the neuronal population in this region decreases markedly; the sleep disturbances of senescence and some aspects of the “sundowning” syndrome (confusion and delirium occurring in the evening) have also been attributed to this cell loss.
Finally, the central role of the hypothalamus in the regulation of both sympathetic and parasympathetic activities must be emphasized. This aspect of hypothalamic function is discussed in the preceding chapter.
The pineal gland, or pineal body, is a small glandular structure (about 9 mm in diameter) that projects from the dorsal diencephalon and lies just posterior to the third ventricle. In the past, the pineal body figured prominently in philosophic and religious writings; for Descartes, it was the seat of the soul. When this idea was discredited, the pineal gland was relegated to the status of a vestigial organ. The identification of melatonin, the pineal hormone—followed by recognition of its role in maintaining biologic rhythms and the modulating effects on its secretion by the circadian light–dark cycle—revived scientific interest in the structure. Even though the hormone was found to have an indirect effect on several other neuroendocrine systems, neurologists took little interest in pineal function because ablation of the gland in humans, with attendant loss of most of its circulating melatonin, leads to few if any clinical changes.
It is the cyclic secretion of melatonin that appears to be the most important activity of the pineal gland. However, melatonin secretion is more accurately regarded as a linked manifestation of the circadian rhythm than as its controlling mechanism. The main cellular element of the gland, the pinealocyte, is thought to be derived from neural photoreceptors in lower vertebrates. The latter cells, structurally analogous to retinal cones, transduce light directly into neural impulses and are among the mechanisms for circadian entrainment of hormonal rhythms. In humans, the pineal no longer possesses the ability to transduce light directly. However, it does retain an input from photic stimuli and influences the circadian light–dark cycle through a pathway that originates in the retina, synapses in the suprachiasmatic nucleus, passes through descending sympathetic tracts to the intermediolateral cell columns and superior cervical ganglia, and then ascends to innervate noradrenergic terminals on the pinealocytes. Darkness elicits a release of norepinephrine from the photoreceptors, stimulating the synthesis and release of melatonin. During daylight the retinal photoreceptor cells are hyperpolarized, norepinephrine release is inhibited, and there is little melatonin production. The concentration of the hormone peaks between 2 and 4 A.M. and gradually falls thereafter. An approximate circadian rhythmicity to melatonin release is preserved in continuous darkness and, inexplicably, the blind maintain a light suppression of secretion. It is readily appreciated that, in humans, it is difficult to separate the changes that occur in the suprachiasmatic nucleus from those of the pineal gland.
Like other neuroendocrine cells, pinealocytes release peptides that are produced in the Golgi apparatus and packaged in secretory granules. Whether secretion is the main mechanism for melatonin release is unclear, as these cells can use an alternative ependymal type of vacuolar secretion. The entire gland is invested by a rich vasculature to receive the released peptide (in some mammals the blood flow per gram of pineal tissue is surpassed only by that of the kidney). The biochemistry and physiology of melatonin have been extensively reviewed by Brzezinski.
In humans, a regular feature of pineal pathology is the accumulation of calcareous deposits in structures termed acervuli (“brain sand”). These have a more complex composition than simply calcium; they are actually composed of carbonate-containing hydroxyapatite that is linked to calcium and other metals. A review of the mineralization of the pineal can be found in the text by Haymaker and Adams. These concretions are formed within vacuoles of pinealocytes and released into the extracellular space. The mineralization of the pineal body provides a convenient marker for its position in plain films and on various imaging studies.
It is of significance that pineal tumors do not secrete melatonin, but the loss of melatonin may be used as a marker for the completeness of surgical pinealectomy. Most interest in the past several years has centered on melatonin as a soporific agent and its potential to reset sleep rhythms. Its concentration in depressive illnesses, especially in the affected elderly, is also decreased. The subject of pineal tumors is discussed later and is contained in Chap. 31, with discussion of other brain tumors.
Hypothalamic Syndromes
Hypothalamic syndromes are of two distinct types (Martin and Reichlin). In one, all or many hypothalamic functions are disordered, often in combination with signs of disease in contiguous structures (“global hypothalamic syndromes,” as described below). The second type is characterized by a selective loss of hypothalamic–hypophyseal function, attributable to a discrete lesion of the hypothalamus and often resulting in a deficiency or overproduction of a single hormone—a partial hypothalamic syndrome.