Diseases of the Spinal Cord: Introduction
Diseases of the nervous system may be confined to the spinal cord where they produce a number of distinctive syndromes. These relate to the special anatomic features of the cord, such as its prominent function in sensorimotor conduction and relatively primitive reflex activity; its long, cylindrical shape; its small cross-sectional size; the peripheral location of myelinated fibers next to the pia; the special arrangement of its blood vessels; and its intimate relationship to the vertebral column. Woolsey and Young estimated that approximately 30 diseases are known to affect the spinal cord, of which half are seen with regularity. These processes express themselves in a number of readily recognized ways and, as will be evident, certain diseases preferentially produce special syndromes. This syndromic grouping of the spinal cord disorders, which is in keeping with the general plan of this book, greatly facilitates clinical diagnosis.
The main syndromes considered in this chapter are (1) a complete or almost complete sensorimotor myelopathy that involves most or all of the ascending and descending tracts (transverse myelopathy); (2) a combined painful radicular and transverse cord syndrome; (3) the hemicord (Brown-Séquard) syndrome; (4) a ventral cord syndrome, sparing posterior column function; (5) a high cervical–foramen magnum syndrome; (6) a central cord or syringomyelic syndrome; (7) a syndrome of the conus medullaris; and (8) a syndrome of the cauda equina. In addition, an important distinction is made between lesions within the cord (intramedullary) and those that compress the cord from without (extramedullary). Some of the anatomic and physiologic considerations pertinent to an understanding of disorders of the cord and of the spine can be found in Chaps. 3, 9 (Figs. 9-5 and 9-7), and Chap. 11, on motor paralysis, somatic sensation, and back pain, respectively. The typical spinal cord syndromes are represented most perfectly by tumor compression that originates in an adjacent vertebral body; this important process is therefore described as a model in the introductory section and again in a later part of the chapter.
The Syndrome of Acute Paraplegia or Quadriplegia Caused by Traumatic and Other Physical Factors (Transverse Myelopathy)
This syndrome is best considered in relation to trauma, its most frequent cause, but it occurs also as a result of other acute damage including infarction or hemorrhage and with rapidly advancing compressive, necrotizing, demyelinative, or inflammatory lesions. Each of these categories of acute spinal cord disease is discussed in the following pages. For convenience we have included in this group radiation myelopathy, which is transverse but evolves subacutely.
Throughout recorded medical history, advances in the understanding of spinal cord disease have coincided largely with periods of warfare. The first thoroughly documented study of the effects of sudden total cord transection was by Theodor Kocher in 1896, based on his observations of 15 patients. During World War I, Riddoch, and later Head and Riddoch, gave what are now considered the classic descriptions of spinal transection in humans; Lhermitte and Guillain and Barré are credited with refining those observations. Little could be done for patients in that era and fully 80 percent died in the first few weeks (from infections); survival was possible only if the spinal cord lesion was partial. World War II marked a turning point in the understanding and management of spinal injuries. The advent of antibiotics and the ability to control skin, bladder, and pulmonary infections permitted the survival of unprecedented numbers of soldiers with cord injuries and provided the opportunity for long-term observation. In special centers, and the care and rehabilitation of the paraplegic patient were brought to a high level. Studies conducted in these centers greatly enhanced our knowledge of the functional capacity of the chronically isolated spinal cord. Kuhn, Munro, Martin and Davis, Guttmann, Pollock, and Pollock and associates made particularly important contributions to this subject.
The usual circumstances of spinal cord injury, in approximate order of frequency in civilian practice are motor vehicle and motorcycle accidents, falls (sometimes during a state of alcoholic intoxication), gunshot or stab wounds, diving accidents, crushing industrial injuries, and birth injury. In the United States, the annual incidence of spinal cord injury has been given as 5 cases per 100,000 population; males predominate (4:1). Each year approximately 3,500 persons die in relation to their spinal injury, and another 5,000 are left with complete or nearly complete loss of spinal cord function.
Although trauma may involve the spinal cord alone, the vertebral column is almost invariably injured at the same time. Often there is an associated cranial injury as pointed out in Chap. 35.
A useful classification of vertebral column injuries divides them into fracture–dislocations, pure fractures, and pure dislocations. The spinal cord that is situated beneath any of these injuries is traumatized by direct compression as a result of dislocation of spinal bones, or by buckling of the ligaments inside the spinal canal. The relative frequency of the three types is about 3:1:1. Except for bullet, shrapnel, and stab wounds, a direct blow to the spine is a relatively uncommon cause of serious spinal cord injury. In civilian life, most fractures and dislocations of the spinal column are the result of force applied at a distance from the site of the disruption of the spinal column. Specifically, all three types of spinal injury are typically produced by a vertical compression of the spinal column, to which either anteroflexion or retroflexion (hyperextension) is added. The most important variables in the mechanics of vertebral injury are the structure of the bones and ligaments at the level of the injury and the intensity, direction, and point of impact of the force. The main elements of the spine are illustrated in Chap. 11.
In keeping with the mechanism of force applied at a distance, blows to the head may result in cervical spinal injuries. If a hard object at high velocity strikes the cranium, a skull fracture occurs, the force of the injury being absorbed mainly by the elastic quality of the skull. If the traumatizing force is relatively soft yet unyielding, or is applied more slowly, the spine, and particularly its most mobile (cervical) portion, will be the part injured. If the neck happens to be rigid and straight and the force is applied quickly to the head, the atlas and the odontoid process of the axis may fracture.
In the case of cervical flexion injury, the head has usually been bent sharply forward when the force is applied. The cervical vertebrae are forced together at the level of maximum stress, driving the anteroinferior edge of the upper vertebral body into the one below, sometimes splitting it in two. The posterior part of the fractured body is displaced backward and compresses the cord. Concomitantly, there is tearing of the interspinous and posterior longitudinal ligaments. Less severe degrees of anteroflexion injury produce only dislocation of adjacent cervical vertebrae at one of several levels. Vulnerability to the effects of anteroflexion (and to some extent to retroflexion injuries) is increased by the presence of cervical spondylosis or ankylosing spondylitis or by a congenital narrowness of the spinal canal.
In cervical hyperextension injuries, the mechanism is one of vertical compression with the head in an extended position. Stress is mainly on the posterior elements (the laminae and pedicles) of the midcervical vertebrae (C4 to C6), or sometimes at higher levels (see the named fractures below), which may be fractured unilaterally or bilaterally, and on the anterior ligaments. This dual disruption in the spinal architecture allows for displacement of one vertebral body upon the adjacent one and compresses the cord between the laminae of the lower vertebra and the body of the one above.
However, spinal cord trauma may also occur from hyperextension injury without apparent damage or misalignment of the vertebrae. In these instances, the spinal cord damage, which can be nonetheless profound and permanent, is considered to be caused by a sudden inward bulge of the ligamentum flavum or by transient vertebral dislocation that is permitted because of ligamentous disruption; when viewed with imaging studies, the vertebral bodies are found to have spontaneously realigned. In such cases, rupture of the supporting ligamentous elements and spinal instability can be revealed by gentle flexion and extension of the neck under radiologic observation, which demonstrates movement of the vertebra in relation to an adjacent one. CT and plain lateral spine films are satisfactory means of demonstrating the vertebral injury but the tearing and bulging of ligaments from vertebral dislocation are more dependably demonstrated by MRI.
Another potential mechanism of cord and spinal root injury involving extremes of extension and flexion of the neck is so-called whiplash or recoil injury, most often the result of an automobile accident. When a vehicle is struck sharply from behind, the head of the occupant is flung back uncontrollably, or if a fast-moving vehicle stops abruptly, there is sudden forward flexion of the neck, followed by retroflexion. Under these conditions the occipitonuchal and sternocleidomastoid muscles and other supporting structures of the neck and head are affected much more often than the spinal cord or roots. Nevertheless, in rare instances, quadriparesis, temporary or permanent, results from a violent whiplash injury. The exact mechanism of neural injury in these circumstances is not clear; perhaps there is a transient posterior dislocation of a vertebral body, a momentary buckling of the ligamentum flavum, or retropulsion of the intervertebral disc into the spinal canal. Other ostensible results of whiplash, such as dizziness, are highly controversial and are discussed in Chap. 11. However, the main comment to be made regarding whiplash is that all manner of neurologic symptoms have been uncritically and inappropriately attributed to it, often with implications for medicolegal and disability determinations.
The presence of a congenitally narrow cervical spinal canal or of acquired spinal diseases such as cervical spondylosis, rheumatoid arthritis, or ankylosing spondylitis adds greatly to the hazard of damage to the cord or roots. Neck trauma of almost any configuration may in particular aggravate preexisting spondylotic symptoms. There are in addition examples of spinal cord compression that result from prolonged static hyperextension of the cervical spine during a protracted period of stupor. This accounts for some cases of quadriplegia following a period of sustained unresponsiveness due to opiate or sedative drug overdose (Ell et al). Arterial hypotension may be an added factor in some instances.
A special type of spinal cord injury, occurring most often in wartime, is one in which a high-velocity missile penetrates the vertebral canal and damages the spinal cord directly. In some cases the missile strikes the vertebral column without entering the spinal canal but disrupts and virtually shatters the intradural contents or produces lesser degrees of spinal cord dysfunction. Or, the transmitted shock wave from a bullet passing nearby the vertebral column causes paralysis of spinal cord function that is largely reversible in a day or two (spinal cord concussion, which is described further on).
Acute traumatic paralysis may also be the indirect consequence of a vascular mechanism, mainly through infarction from fibrocartilaginous emboli arising in an intervertebral disc that has ruptured into a radicular artery or vein of the cord. Or a traumatic dissecting aneurysm of the aorta may occlude the segmental arteries of the spinal cord, as in the cases reported by Weisman and Adams and by Kneisley. One striking variant of this type of vascular injury is infarction of the upper cervical cord, resulting in hemi-, tri-, or tetraplegia, from dissection of one or both vertebral arteries and occlusion of their tributary anterior spinal arteries at the cervicomedullary junction.
An analysis from a former era of 2,000 cases of spinal injury collected from the medical literature by Jefferson up to 1927 is still valid and showed that most vertebral injuries occurred at the levels of the first and second cervical, fourth to sixth cervical, and eleventh thoracic to second lumbar vertebrae. Industrial accidents most often involved the thoracolumbar vertebrae. Impact to the head with the neck flexed or sharply retroflexed, as mentioned earlier, was the main cause of injuries to the cervical region. These are not only the most mobile portions of the vertebral column but also the regions in which the cervical and lumbar enlargements of the cord greatly reduce the space between neural and bony structures. The thoracic cord is relatively small and its spinal canal is capacious; additional protection is provided by the high and overlapping articular facets, making dislocation difficult, and limitations in anterior displacement of vertebral bodies imposed by the thoracic cage.
Several configurations of vertebral fractures are common enough that they are designated by eponyms or descriptive terms. The knowledgeable clinician has some familiarity with them. They are summarized in Table 44-1. They include the Jefferson fracture, hangman’s fracture, the Chance fracture, atlanto-axial (C1-C2) and the more common atlanto-occipital fracture-dislocation, including fracture of the dens of C-2. Regarding hangman’s fracture, contrary to the popular notion, most penal hangings do not cause vertebral bony disruption and death is instead by strangulation; a more common mechanism for hangman’s fracture is an elderly person who falls and strikes the chin, causing hyperextension of the neck. The majority of fatal cases of cervical spine injury are from fracture–dislocations of the upper cervical spine (C1 to C3 vertebrae, thus encompassing atlanto-occipital and atlanto-axis dislocations with sudden respiratory paralysis).
NOMENCLATURE | MECHANISM | IMAGING | STABILITY | CLINICAL EFFECTSa |
|---|---|---|---|---|
Atlanto-occipital dislocation | Rotatory force to head | Displacement of occipital condyles in relation to lateral masses of C1 | Unstable | Common in children; fatal if severe |
Atlanto-axial dislocation | Rotatory mechanism common in children; flexion in adults | Dislocation of C1-C2 facet | Unstable | Varies from asymptomatic to severe myelopathy |
Jefferson fracture (C1) | Axial downward force on vertex of head | Bilateral anterior and posterior arch fractures | Stable | Usually asymptomatic; transverse ligament may be disrupted |
Odontoid (dens) fractures (C2) | Hyperflexion | Fracture through C2b: Type 1: tip of dens Type 2: base of dens Type 3: body of C2 | ||
Type 2 most “unstable” and unlikely to heal spontaneously | Varies from asymptomatic to tetraparesis | |||
Hangman’s fracture (C2) | Hyperextension with axial loading | Fractures through pedicles of C2 | Usually stable | Most are asymptomatic |
Subaxial fracture- dislocation | Severe flexion | Dislocation (perched or jumped) of facets with reversal of normal “shingled” appearance | Poor | Occurs at any level C3 to T1; common cause of traumatic tetra- and quadriplegia; vertebral artery dissection |
Burst fracture (thoracolumbar) | Axial loading | Fracture through vertebral body with loss of height | Variable | Root compression from retropulsion of bone fragment |
Chance fracture (thoracolumbar) | Flexion of lower thoracic spine —”seat-belt” injury | Same as burst fracture but includes fractures through facets and posterior elements | Variable | Commonly asymptomatic |
Compression (wedge) fracture (thoracolumbar) | Hyperflexion | Wedging of anterior vertebral body, no loss of height and no subluxation | Usually stable | Local pain, rarely neurologic deficit |
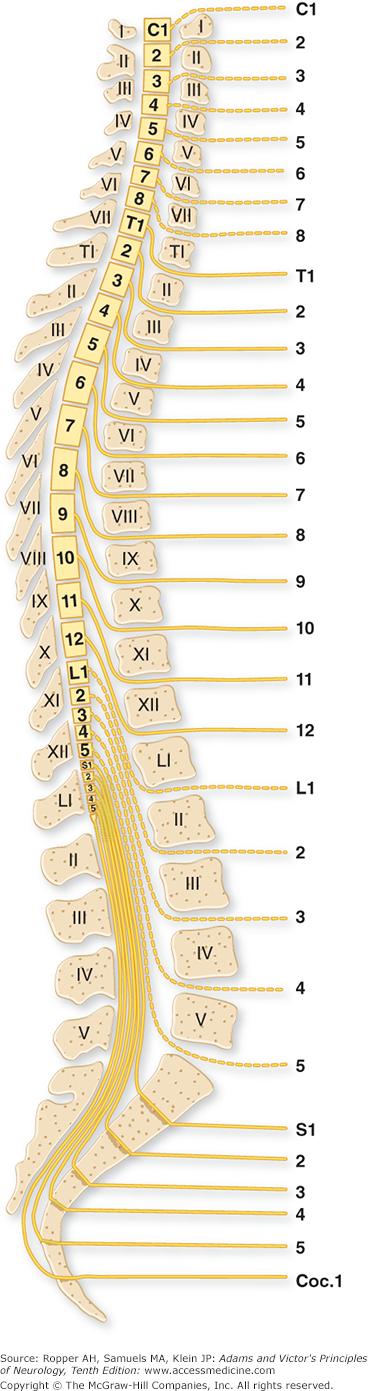
The level of the spinal cord damage and, by implication, the level of disruption of the spinal column, can be determined from clinical findings. Diaphragmatic paralysis occurs with lesions of the upper three cervical segments (transient arrest of breathing from brainstem paralysis is common in severe head injury). Complete paralysis of the arms and legs usually indicates a fracture or dislocation at the fourth to fifth cervical vertebrae. If the legs are paralyzed and the arms can still be abducted and flexed, the lesion is likely to be at the fifth to sixth cervical vertebrae. Paralysis of the legs and only the hands indicates a lesion at the sixth to seventh cervical level. Below the cervical region, the spinal cord segments and roots are not directly opposite their similarly numbered vertebrae (Fig. 44-1). The spinal cord ends at the first lumbar vertebra, usually at its rostral border. Vertebral column lesions below this point give rise predominantly to cauda equina syndromes; these carry a better prognosis than injuries to the lower thoracic vertebrae, which involve both cord and multiple roots.
Figure 44-1.
The relationship of spinal segments and roots to the vertebral bodies and spinous processes. The cervical roots (except C8) exit through foramina above their respective vertebral bodies, and the other roots issue below these bodies. (From Haymaker and Woodhall: Peripheral Nerve Injuries, 2nd ed. Philadelphia, Saunders, 1953, by permission.)
The level of sensory loss on the trunk, as determined by perception of pinprick, is an accurate guide to the level of the lesion, with a few qualifications. (See Figs. 9-1, 9-3, and 9-4 for maps of the sensory dermatomes.) Lesions of the lower cervical cord, even if complete, may spare sensation down to the nipple line because of the contribution of the C3 and C4 cutaneous branches of the cervical plexus, which variably innervate skin below the clavicle. Or a lesion that involves only the outermost fibers of the spinothalamic pathways results in a sensory level (to pain and temperature) well below the level of the lesion. In all cases of spinal cord and cauda equina injury, the prognosis for recovery is more favorable if any movement or sensation is elicitable during the first 48 to 72 h.
If the spinal column can be examined safely, it should be inspected and palpated for angulations or irregularities and gently percussed to detect underlying bony injury. Collateral injury of the thorax, abdomen, and long bones should be sought and cranial injury is a concern if the mechanism of direct spinal impact is not known from the history.
A neurologic examination with recording of motor, sensory, and sphincter function is necessary to follow the clinical progress of spinal cord injury. A common practice is to define the injury according to the standards of the American Spinal Injury Association and to assign the injury to a point on the ASIA Impairment, or AIS (a derivative of the formerly used Frankel scale). A paraphrased version that we have found useful is presented here with comments regarding functional ability from the Frankel scale:
Complete: no sensory or motor function below the level of the lesion including in the sacral segments
Sensory incomplete: sensory function is preserved but motor function is lost below the zone of injury
Motor incomplete (first grade): motor function is reduced in more than half of key muscles below the level of the lesion; this usually renders the patient unable to walk. (Reduced motor function is defined as active movement in a full range of motion only if gravity is eliminated.)
Motor incomplete (second grade): motor function is reduced in fewerthan half of key muscles below the level of the lesion; this usually allows standing and walking
Normal: reflexes may be abnormal
Obviously, groups C, D, and E have a more favorable prognosis for recovery of ambulation than does groups A and B.
In cases of suspected spinal injury, the immediate concern is that movement (especially flexion) of the cervical spine be avoided. The patient should ideally be placed supine on a firm, flat surface (with one person assigned, if possible, to keeping the head and neck immobile) and should be transported by a vehicle that can accept the litter. The board may be placed under the patient, gently rolling him to one side with the head, neck, and body held in alignment. If moving the patient is not feasible, the neck may be immobilized in place with a form collar or an equivalent device that is contrived at the scene, or even the examiner’s hands held firmly along the cervical spine. The patient should ideally be transported by an ambulance equipped with spine boards, to which the head is fixed by straps. This provides a more effective means of immobilization than sandbags or similar objects placed on each side of the head and neck. On arrival at the hospital, it is prudent to have the cervical spine remain immobilized until a lateral film or a CT or MRI of the cervical spine can been obtained, with the qualifications below.
Several schemes have been devised for determining which patients may require imaging; these are comparable to “rules” for the use of imaging in head injury that are discussed in Chap. 35. The two more widely cited ones for spinal injury are those of the NEXUS group (Hoffman et al) and the “Canadian C-spine Rule” (Stiell et al). The former identifies those at low risk for spinal cord injury on the basis of the absence of posterior midline cervical-spine tenderness, no evidence of intoxication, a normal level of alertness (thereby allowing accurate reporting of the circumstances of injury and the presence of neck pain, and suggesting there has not been serious brain injury), no focal neurologic deficit, and no other painful injuries that distract the patient from reporting neck pain. The Canadian rule has been found to be slightly more sensitive and specific (this has been disputed); it is based on three high-risk criteria: age older than 65 years, a dangerous mechanism of injury, and limb paresthesias; and on any of six features that are associated with low-risk of cord injury: simple rear-end motor vehicle collision, sitting position in the emergency department, being ambulatory at any time after injury, delayed (not immediate) onset of neck pain, absence of midline cervical-spine tenderness, coupled with the ability to turn the head 45 degrees in both directions without pain.
As a result of squeezing or shearing of the spinal cord, there is destruction of gray and white matter and a variable amount of hemorrhage, chiefly in the more vascular central parts. These changes, designated as traumatic necrosis of the cord, are maximal at the level of injury and at one or two segments above and below it. Rarely is the cord cut in two, and seldom is the pia-arachnoid lacerated. Separation of the components of traumatic necrosis, such as hematomyelia, concussion, contusion, and hematorrhachis (bleeding into the spinal canal) is not of great value either clinically or pathologically. As the lesion heals, it leaves a gliotic focus or cavitation with variable amounts of hemosiderin and iron pigment. Progressive cavitation (traumatic syringomyelia) may develop after an interval of months or years and, as the cavity enlarges beyond the main lesion, lead to a delayed central or incomplete transverse cord syndrome. In some instances, the lesion is virtually restricted to the centrally situated gray matter, giving rise to segmental weakness and sensory loss in the arms with few long tract signs. This is the central cervical cord syndrome, also called the Schneider syndrome (see further on). Fragments of the central cord syndrome commonly occur as transient phenomena that reverse over several days.
Investigation of the pathophysiology of acute spinal cord injury dates from the experimental studies of Allen in the early 1900s. His method consisted of dropping graded weights onto the dura-covered thoracic cord of surgically prepared animals. The technique was refined over the years by precise measurements of the velocity, force, and direction of the dropped weights. This type of impact on the cord, of sufficient severity to render the animal immediately paraplegic and abolish sensory-evoked responses from structures below the lesion, indicates that action potentials can no longer be conducted across the injured spinal cord segment. No histologic changes, by either light or electron microscopy, can be detected for several minutes after impact. The earliest tissue alterations consist of hyperemia and small hemorrhages in the central gray matter. By 1 h, the microscopic hemorrhages coalesce and become macroscopically visible. Tissue oxygen saturation is diminished in the region. Within 4 h, the central part of the cord swells and a spreading edema pervades the surrounding white matter; however, necrosis may not be evident for up to 8 h, an observation that has led to numerous strategies designed to spare the neurons and long tracts. Surgical intervention to minimize white matter edema—such as laminectomy and myelotomy—spinal cord cooling, hyperbaric exposure, and the administration of pharmacologic measures have been tried but, for the most part, have had no meaningful effects on the evolving lesion.
Certain mechanisms that are thought to be operative in the death of cerebral neurons exposed to ischemia or to traumatic forces have also been invoked in spinal cord injury but with limited evidence to support this commonality. These include release of excitotoxins such as glutamate and exposure of neurons to calcium and free radicals. Despite early experiments implicating neurotransmitters or opioid-like substances, later work failed to substantiate this or other similar secondary mechanisms. One problem with all the experimental work is that it only imperfectly reproduces spinal injury in humans. Most recent work in the field of spinal cord injury has been on regeneration of spinal tissue across gaps in the cord using stem cells, gene therapy, and tissue scaffolding made of artificial or in vitro cell structures. None has yet proved satisfactory for clinical implementation.
When the spinal cord is suddenly and severely impacted, three disorders of function are at once evident: (1) all voluntary movement in parts of the body below the lesion is immediately lost; (2) sensation from the lower parts of the body is abolished; and (3) reflex functions in segments of the isolated spinal cord are suspended. The last effect, termed spinal shock, involves tendon as well as autonomic reflexes. This state is of variable duration (1 to 6 weeks but sometimes far longer) and is so dramatic that Riddoch used it as a basis for dividing the clinical effects of spinal cord transection into two stages, that of spinal shock with areflexia followed by a stage of heightened reflex activity.
The separation of these stages is not as sharp as this statement might imply. Less complete or less sudden lesions of the cord result in little or no spinal shock. The features of complete functional spinal cord transection are now presented in detail because of their practical value and the special place they occupy in classic neurology.
The loss of motor function at the time of injury, tetraplegia with lesions of the fourth to fifth cervical segments or above, and paraplegia with lesions of the thoracic cord, are accompanied by immediate atonic paralysis of bladder and bowel, gastric atony, loss of sensation below a level corresponding to the spinal cord lesion, muscular flaccidity, and almost complete suppression of spinal segmental reflex activity below the lesion. As a result of their sudden separation from higher levels of control, the neural elements below the lesion essentially fail to perform their normal function. As dramatic as this state of reflex paralysis is, its physiologic basis is incompletely understood. Also impaired in the segments below the lesion is the control of autonomic function. Vasomotor tone, sweating, and piloerection in the lower parts of the body are temporarily abolished. As a result, there may be severe systemic hypotension that itself contributes to spinal cord damage. The lower extremities lose heat if left uncovered, and they swell if dependent. The skin over time becomes dry and pale, and ulcerations may develop over bony prominences. The sphincters of the bladder and the rectum remain contracted to some degree because of the loss of the normal inhibitory influence of higher centers, but the detrusor of the bladder and smooth muscle of the rectum become atonic. Urine accumulates until the intravesicular pressure is sufficient to overcome the sphincters, causing overflow incontinence. There is also passive distention of the bowel, retention of feces, and absence of peristalsis (paralytic ileus). Genital reflexes (penile erection, bulbocavernosus reflex, contraction of dartos muscle) are abolished or profoundly depressed.
The duration of the stage of spinal shock varies considerably. In a small number (5 of Kuhn’s 29 patients, for example) it is permanent, or only fragmentary reflex activity is regained, even many years after the injury. In such patients, the spinal segments below the level of transection may have themselves been injured, perhaps by a vascular mechanism, although this explanation is unproven. More likely there is a loss of the brainstem–spinal facilitatory mechanisms and an increase in inhibitory activity in the isolated segments. In other patients, minimal genital and flexor reflex activity can be detected within a few days of the injury and minimal reflex activity appears within a period of 1 to 6 weeks. Usually the bulbocavernosus reflex is the first to return. Contraction of the anal sphincter can be elicited by plantar or perianal stimulation, and other genital reflexes reappear at about the same time. The F-waves, electrophysiologic responses that reflect the functioning of the motor neurons of the isolated segment of the cord, are suppressed until spasticity supervenes, at which time they become overly easy to elicit. Noxious stimulation of the plantar surfaces evokes a tremulous twitching and brief flexion or extension movements of the great toes.
The explanation of spinal shock, which is brief in sub-mammalian animals and more lasting in higher mammals, especially in primates, is believed to be the sudden interruption of suprasegmental descending fiber systems that normally keep the spinal motor neurons in a continuous state of readiness. In the cat and monkey, Fulton found the facilitatory tracts in question to be the reticulospinal and vestibulospinal. Subsequent studies showed that in monkeys, some degree of spinal shock could result from interruption of the corticospinal tracts alone. This is probably not a significant factor, however, at least in humans, because spinal shock does not result from acute cerebral and brainstem lesions that interrupt the corticospinal tracts.
This is the more familiar condition of spasticity that emerges some time after spinal injury and is also typical of most of the nontraumatic subacute myelopathies that have developed more slowly than traumatic injuries and have not had a period of spinal shock. A few weeks after an acute traumatic injury, all reflex responses, which are initially minimal and unsustained, become stronger and more easily elicitable and as time passes, come to include additional and more proximal muscles. Gradually, the typical pattern of heightened flexion reflexes emerges: dorsiflexion of the big toe (Babinski sign); fanning of the other toes; and later, flexion or slow withdrawal movements of the foot, leg, and thigh with contraction of the tensor fascia lata muscle (the last several features referred to as “triple flexion”). Tactile stimulation of the foot may suffice as a stimulus, but a painful stimulus is more effective. The Achilles reflexes and then the patellar reflexes return. Retention of urine becomes less complete, and at irregular intervals urine is expelled by spontaneous contractions of the detrusor muscle. Reflex defecation also begins. After several months the withdrawal reflexes become greatly exaggerated, to the point of flexor spasms, and they may be accompanied by profuse sweating, piloerection, and automatic emptying of the bladder (occasionally of the rectum). This is the “mass reflex,” which can be evoked by stimulation of the skin of the legs or by some interoceptive stimulus, such as a full bladder. Varying degrees of heightened flexor reflex activity may last for years, or indefinitely. Heat-induced sweating is defective, but reflex-evoked (“spinal”) sweating may be profuse (see Kneisley). In such cases the lateral horn cells in much of the thoracic cord are still viable and have been disinhibited. Above the level of the lesion, thermoregulatory sweating may be exaggerated in order to compensate for the loss of evaporative cooling of lower segments, and there is cutaneous flushing, hypertension that causes pounding headache and reflex bradycardia. This syndrome (“autonomic dysreflexia”) is episodic and occurs in response to a certain stimuli, such as a distended bladder or rectum. It has been ascribed to the reflex release of adrenaline from the adrenal medulla and of norepinephrine from the disinhibited sympathetic terminals caudal to the lesion but is exaggerated by defective baroreceptor compensatory reflexes as discussed in Chap 27.
Extensor reflexes and tone eventually develop in most cases (18 of 22 of Kuhn’s patients who survived more than 2 years), but their appearance does not lead to the abolition of the exaggerated flexor reflexes. The overactivity of extensor muscles may appear as early as 6 months after the injury, but this only happens, as a rule, after the flexor responses are fully developed. Extensor responses are at first manifest in certain muscles of the hip and thigh and later of the leg. In a few patients, extensor reflexes are organized into support reactions sufficient to permit spinal standing. Kuhn observed that extensor movements were at first provoked most readily by a sudden shift from a sitting to a supine position and later by proprioceptive stimuli (squeezing of the thigh muscles) and tactile stimuli from wide areas. Marshall, in a study of 44 patients with chronic spastic paraplegia of spinal origin, found all possible combinations of flexor and extensor reflexes; the type of reflex obtained was determined by the intensity and duration of the stimulus (a mild prolonged noxious stimulus evoked an ipsilateral extensor reflex; an intense brief stimulus, a flexor response).
From these observations one would suspect that the ultimate posture of the legs—flexion or extension—does not depend solely on the completeness or incompleteness of the spinal cord lesion, as originally postulated by Riddoch. The development of paraplegia in flexion (extreme flexion of the hips and knees, as in a fetal position) relates also to the level of the lesion, being seen most often with cervical lesions and progressively less often with more caudal ones. Greatly troubling to the spinal patient are repeated flexor spasms, which are more frequent with higher lesions of the cord, and the ensuing contractures ultimately produce a fixed flexor posture. Furthermore, reduction of flexor spasms by elimination of nociceptive stimuli (infected bladder, decubitus, etc.) favors an extensor posture of the legs (paraplegia in extension). According to Guttmann, the positioning of the limbs during the early stages of paraplegia influences their ultimate posture. Thus, prolonged fixation of the paralyzed limbs in adduction and semiflexion favors subsequent paraplegia in flexion. Placing the patient prone or placing the limbs in abduction and extension facilitates the development of predominantly extensor postures. Nevertheless, strong and persistent extensor postures are usually observed only with partial lesions of the spinal cord.
A number of sensory phenomena are expected after functional cord transection. The main one, of course, is the loss of all sensibility below the lesion, i.e., the sensory level. Of some interest is the fact that many patients report sensory symptoms in segments of the body below the level of their transection. Thus, a tactile stimulus above the level of the lesion may be felt below the transection (a type of synesthesia). Patients describe a variety of paresthesias, the most common being a dull, burning pain in the lower back and abdomen, buttocks, and perineum. We have encountered several patients in whom aching testicular or rectal pain was a very distressing problem. The pain may be intense and last for a year or longer, after which it gradually subsides. It persists after rhizotomy but can be abolished by anesthetizing the stump of the proximal (upper) segment of the spinal cord, according to Pollock and coworkers. Transmission of sensation over splanchnic afferents to levels of the spinal cord above the lesion, the conventional explanation, is therefore not the most plausible one.
The overactivity of sensory systems in the isolated segments of the spinal cord has several explanations. One assumes that suprasegmental inhibitory influences have been removed by the transection, so that afferent sensory impulses evoke exaggerated nocifensive and phasic and tonic myotatic reflexes. But isolated neurons also become hypersensitive to neurotransmitters. Since the early experiments of Cannon and Rosenblueth, it has been known that section of sympathetic motor fibers leaves the denervated structures hypersensitive to epinephrine and to acetylcholine.
Various combinations of residual deficits (of lower and upper motor neurons and sensory neurons) are to be expected. High cervical lesions, for example, may result in extreme and prolonged tonic spasms of the legs as a result of release of tonic myotatic reflexes. Under these circumstances, attempted voluntary movement may excite intense contraction of all flexor and extensor muscles lasting for several minutes. Segmental damage in the low cervical or lumbar gray matter, destroying inhibitory Renshaw neurons, may release activity of remaining anterior horn cells, leading to spinal segmental spasticity.
Any residual symptoms persisting after 6 months are likely to be permanent, although in a small proportion of patients some return of function (particularly sensation) is possible after this time. Loss of motor and sensory function above the lesion, coming on years after the trauma, is the result of an enlarging cavity in the proximal segment of the cord (see further on, under “Syringomyelia [Syrinx]”).
These terms refer to a transient loss of motor sensory function of the spinal cord that recovers within minutes or hours but may persist in mild form for days or more. In most instances, the symptoms are rapidly diminishing and few neurologic abnormalities are found at the time of the first examination. There are a number of such transient syndromes: bibrachial weakness; quadriparesis (occasionally hemiparesis); paresthesias and dysesthesias in a similar distribution to the weakness; or sensory symptoms alone (“burning hands syndrome”). In the first and last of these, transient dysfunction of the central gray matter of the cervical cord is implicated. It is assumed that the cord undergoes some form of elastic deformation when the cervical spine is compressed or hyperextended; however, the same effects can be produced by direct blows to the spine or forceful falls flat on the back and occasionally, by a sharp fall on the tip of the coccyx. Little is known of the physiologic mechanisms that underlie these reversible syndromes.
Spinal cord concussion from direct impact is observed most frequently in athletes engaged in contact sports (football, rugby, and hockey). An incomplete and reversible myelopathy is referable to the site and level of the injury. A congenitally narrow cervical canal is thought to predispose to spinal cord concussion and to increase the risk of recurrence. As with cerebral concussion, particularly if there have been previous concussions, a difficult decision arises—whether or not to allow resumption of competitive sports. There are no reliable data on which to base this decision, only guidelines that tentatively allow continued participation, after an unspecified period of rest, if the deficit has been brief. It is, however, advisable in most cases to be certain that spinal instability has not been induced by the injury. This can be ascertained from flexion and extension X-ray images of the affected spinal region. The subject is reviewed by Zwimpfer and Bernstein. In athletic contact injury, unilateral arm and hand paresthesias are more common than symptoms of both arm, but they are usually from stretching of the brachial plexus on one side (a “stinger”), rather than from a cord injury.
A special from of acute cervical cord injury implicates mainly central cord damage, resulting in the loss of motor function solely or more severely in the upper limbs than in the lower ones, and it particularly affects the hands. Bladder dysfunction with urinary retention occurs in some cases and sensory loss is often slight (hyperpathia over the shoulders and arms may be the only sensory abnormality). Many of these instances are reversible but damage to the centrally situated gray matter may leave an atrophic, areflexic paralysis of the arms and hands and a segmental loss of pain and thermal sensation from interruption of crossing pain and thermal fibers. Retroflexion injuries of the head and neck are the ones most often associated with the central cord syndrome, but other causes include hematomyelia, fibrocartilaginous embolism, and infarction from dissection of the vertebral artery in the medullary-cervical region as mentioned earlier in the chapter (see Morse for further discussion).
According to Dickman and colleagues, approximately 4 percent of patients who survive injuries of the very rostral cervical cord demonstrate a very limited form of the central cord syndrome, recognized by Nielson and named by Bell, “cruciate paralysis.” The weakness is very selective, being practically limited to the arms, a feature that is attributable to the segregation within the pyramidal decussation of corticospinal fibers to the arms (being rostral) and to the legs (more caudally situated). The arm weakness may be asymmetrical or even unilateral and sensory loss is inconsistent. The patients described have had contusions of the C1-C2 region. Whether the lesion lies strictly within the decussating corticospinal tract or involves central gray matter is not always clear; MRI findings have implicated the latter, as described by Inamasu et al.
For some time, many centers administered methylprednisolone in high dosage (bolus of 30 mg/kg followed by 5.4 mg/kg every hour), beginning within 8 h of the injury and continued for 23 h. This measure, according to the multicenter National Acute Spinal Cord Study (Bracken et al, 1990) resulted in a slight improvement in both motor and sensory function. The therapeutic value of this measure has since been questioned after reanalysis of the data (Nesathurai; Hurlbert) and it is no longer considered essential. Hypotension is treated with infusions of normal saline and may require the transient use of pressor agents. The use of hypothermia with cooling blankets or the infusion of cooled saline is under investigation to protect spinal tissue but has not been validated.
Next, imaging examinations are undertaken to determine the alignment of vertebrae and pedicles, fracture of the pedicle or vertebral body, compression of the spinal cord or cauda equina as a consequence of malalignment, or bone debris in the spinal canal, and the presence of tissue damage within the cord. MRI is ideally suited to display these processes, but if it is not available, myelography with CT scanning is an alternative. Instability of the spinal elements can often be inferred from dislocations or from certain fractures of the pedicles, pars articularis, or transverse processes, but gentle flexion and extension of the injured areas must sometimes be undertaken and plain films obtained in each position.
If a cervical spinal cord injury is associated with vertebral dislocation, traction on the neck may be necessary to secure proper alignment and maintain immobilization. Depending on the nature of the injury, this is accomplished by use of a halo brace, which, of all the appliances used for this purpose provides the most rigid external fixation of the cervical spine. This type of fixation is usually continued for 4 to 6 weeks, after which a rigid collar may be substituted.
Concerning the early surgical management of spinal cord injury, there have traditionally been two perspectives. One, represented by Guttmann and others, advocated reduction and alignment of the dislocated vertebrae by traction and immobilization until skeletal fixation is obtained, and then rehabilitation. The other approach, represented by Munro and later by Collins and Chehrazi, proposed early surgical decompression, correction of bony displacements, and removal of herniated disc tissue and intra- and extramedullary hemorrhage; often the spine is fixed at the same time by a bone graft or other form of stabilization. The issue of acute decompressive surgery remains contentious to the present day. The MRI has altered these empirical approaches by allowing the early demonstration of hematomas and other sources of compression that may be amenable to surgery. With clinical evidence of a complete spinal cord lesion, most surgeons do not favor early surgery.
The results of the conservative and aggressive surgical plans of management for incomplete cord injuries have been difficult to compare and have not been evaluated with modern neurologic techniques. Collins, a participant in the National Institutes of Health (NIH) study of acute management of spinal cord injury 20 years ago, concluded that the survival rate was increased as a result of early surgical stabilization of fractures and fixation of the spine. Others, however, have not been able to document a reduction in neurologic disability and have increasingly been inclined toward nonoperative management of both complete and partial spinal cord lesions (see, for example, Clark; Murphy et al). Many North American neurosurgeons take the less aggressive stance, delaying operation or operating only on patients with compound wounds or those with progression or worsening of the neurologic deficit despite adequate reduction and stabilization. In each case, the approach is guided by the specific aspects of the injuries; ligamentous disruption, presence of hematoma, misalignment-displacement of spinal segments, instability of the injury, and fracture type.
The greatest risks to the patient with spinal cord injury occur in the first 10 days when gastric dilatation, ileus, shock, and infection are threats to life. According to Messard and colleagues, the mortality rate falls rapidly after 3 months; beyond this time, 86 percent of paraplegics and 80 percent of quadriplegics will survive for 10 years or longer. In children, the survival rate is even higher according to DeVivo and colleagues, who found that the cumulative 7-year survival rate in spinal cord–injured children (who had survived at least 24 h after injury) was 87 percent. Advanced age at the time of injury and being rendered completely quadriplegic were the worst prognostic factors.
The aftercare of patients with paraplegia, in addition to substantial psychological support to allow accommodation to new limitations while encouraging a productive life, is concerned with management of bladder and bowel disturbances, care of the skin, prevention of pulmonary embolism, and maintenance of nutrition. Decubitus ulcers can be reduced by frequent turning to avoid pressure necrosis, use of special mattresses, and meticulous skin care. Deep decubitus lesions require debridement and full-thickness grafting. At first, continual catheterization is necessary; then, after several weeks, the bladder can be managed by intermittent catheterization once or twice daily, using a scrupulous aseptic technique. Close surveillance is needed for bladder infection, which is treated promptly should it occur. Bacteriuria alone is common and does not require treatment with antibiotics unless there is associated pyuria. Morning suppositories and periodically spaced enemas are effective means of controlling fecal incontinence. Chronic pain (present in 30 to 50 percent of cases) requires the use of nonsteroidal antiinflammatory medication, injections of local anesthetics, and transcutaneous nerve stimulation. A combination of carbamazepine or gabapentin and either clonazepam or tricyclic antidepressants may be helpful in cases of burning leg and trunk pain. Remaining pain may require more aggressive therapy, such as epidural injections of analgesics or corticosteroids or an implanted spinal cord stimulator that is applied to the dorsal columns or an analgesic pump, but often even these measures are ineffective. Fentanyl transcutaneous patches may be tried. Spasticity and flexor spasms may be troublesome; oral baclofen, diazepam, or tizanidine may provide some relief. In permanent spastic paraplegia with severe stiffness and adductor and flexor spasms of the legs, intrathecal baclofen, delivered by an automated pump in doses up to 400 mg/d, has also been helpful. The drug is believed to act at the synapses of spinal reflexes (Penn and Kroin). Selective injection of botulinum toxin may provide relief of some spastic deformities and of spasms. One must always be alert to the threat of pulmonary embolism from deep-vein thrombi, although the incidence is surprisingly low after the first several months. Physical therapy, muscle reeducation, and the proper use of braces are all important in the rehabilitation of the patient. All this is best carried out in special centers.
Delayed necrosis of the spinal cord and brain are recognized sequela of radiation therapy for tumors in the thorax and neck. Mediastinal irradiation for Hodgkin disease or for other lymphomas is a typical setting for the development of these complications up to decades later. A lower motor neuron syndrome, presumably a result of injury to the gray matter of the spinal cord, may also follow radiation therapy in which the cord was inside the zone of treatment, as described below.
An “early” type of radiation myelopathy (appearing 3 to 6 months after radiotherapy) is characterized mainly by spontaneous uncomfortable sensations in the extremities. The paresthesias may be evoked by neck flexion (Lhermitte symptom). In one of our patients there was impairment of vibratory and position sense in the legs, but no weakness or signs of spinothalamic tract damage. The sensory abnormalities disappear after a few months and, according to Jones, are not followed by the delayed progressive radiation myelopathy described below. The pathology of the early and transient radiation myelopathy has not been fully elucidated, but there is a spongy appearance of the white matter with demyelination and depletion of oligodendrocytes.
This is one of the most dreaded complications of radiation therapy. It is a progressive myelopathy that follows, after a variable latent period, the radiation of malignant lesions in the vicinity of the spinal cord. The incidence of this complication is difficult to determine because many patients die of their malignant disease before the myelopathy has fully evolved but it is estimated to be between 2 and 3 percent (Palmer). According to Douglas and colleagues, patients who have undergone hyperthermia as an adjunctive treatment for cancer are particularly vulnerable to radiation myelopathy.
The neurologic disorder first appears 6 months or more after the course of radiation therapy, usually between 12 and 15 months (latent periods as long as 60 months or longer have been reported). The onset is insidious, usually with sensory symptoms—paresthesias and dysesthesias of the feet or a Lhermitte phenomenon, and similar symptoms in the hands in cases of cervical cord damage. Weakness of one or both legs usually follows the sensory loss. Initially, local pain is absent, in distinction to the effects of spinal metastases. In some cases, the sensory abnormalities are transitory as in the syndrome described above; more often, additional signs make their appearance and progress, at first rapidly and then more slowly and irregularly, over a period of several weeks or months, with involvement of the corticospinal and spinothalamic pathways. The neurologic disturbance may take the form of a Brown-Séquard syndrome, but with progression it is usually overtaken by a transverse myelopathy.
Reagan and coworkers, who have had considerable experience with this condition, described yet another myelopathic radiation syndrome, namely, a slowly evolving amyotrophy, with weakness and atrophy of muscles and areflexia in parts of the body supplied by anterior horn cells of the irradiated spinal segments. Most patients with this form of the disease die within a year of onset. Knowledge of the pathology is incomplete. This syndrome is reminiscent of the delayed motor neuron myelopathy following electrical or lightning injury described in the next section. There is also an unusual paraneoplastic variety of poliomyelopathy and an even less common necrotic myelopathy, mentioned below and in Chap. 31.
The CSF in delayed progressive radiation transverse myelopathy is normal except for a slight elevation of protein content in some cases. MRI of the affected segments of cord demonstrates abnormal signal intensity, decreased in T1-weighted and increased in T2-weighted images. Early in the course of the myelopathy the cord may be swollen, and there is often heterogeneous enhancement with gadolinium infusion. The location of the lesion corresponds to the irradiated portal, which can be identified by the radiation effect on the marrow of the overlying vertebral bodies. The spinal cord lesion tends to be more extensive in rostral–caudal dimension than the usual vascular or demyelinative myelopathy. These are important points to establish, because a mistaken diagnosis of intraspinal tumor or of a dural arteriovenous fistula may lead to an unnecessary operation or further irradiation.
Corresponding with the level of the radiated area and extending over several segments, there is an irregular zone of coagulation necrosis involving both white and gray matter, the former to a greater extent than the latter. Varying degrees of secondary degeneration are seen in the ascending and descending tracts. Vascular changes—necrosis of arterioles or hyaline thickening of their walls and thrombotic occlusion of their lumens—are prominent in the most severely damaged portions of the cord. Most neuropathologists have attributed the parenchymal lesion to the blood vessel changes; others believe that the degree of vascular change is insufficient to explain the necrosis (Malamud et al; Burns et al). Certainly, the most severe changes in the cord are consistent with infarction, but the insidious onset and slow, steady progression of the disorder and the coagulative nature of the necrosis would then have to be explained by a steady succession of vascular occlusions. Exceptional instances, in which a transverse myelopathy has developed within a few hours of radiation treatment (as described by Reagan et al), are more readily explained by thrombotic occlusion of a large spinal artery.
Neurologists associated with cancer treatment centers are sometimes confronted with a patient who exhibits the late development (up to 10 to 15 years after radiation) of a slowly progressive sensorimotor paralysis of only one limb (motor weakness predominates) or one region of the body. This usually represents damage in the peripheral nervous system. Examples that we have encountered are multiple cranial neuropathies after radiation of nasopharyngeal tumors, cervical and especially brachial neuropathies after laryngeal and breast cancers, and lumbosacral plexopathies and cauda equina damage with pelvic radiation. These are discussed further in Chap. 46, on diseases of the peripheral nerves.
Kagan and colleagues have determined the tolerance of the adult human spinal cord to radiation, taking into account the volume of tissue irradiated, the duration of the irradiation, and the total dose. They reviewed all of the cases in the literature up to 1980 and concluded that radiation injury could be avoided if the total dose was kept below 6,000 cGy and was given over a period of 30 to 70 days, provided that each daily fraction did not exceed 200 cGy and the weekly dose was not in excess of 900 cGy. It is noteworthy that in the cases reported by Sanyal and associates, the amount of radiation surpassed these limits. Forewarned with this knowledge, radiation specialists have the impression that the incidence of this complication is decreasing. Of course, if the underlying neoplasm is likely to be imminently fatal, palliative radiation can exceed these limits.
A number of case reports remark on temporary improvement in neurologic function after the administration of corticosteroids. This therapy should be tried because in some patients it appears to arrest the process short of complete destruction of all sensory and motor tracts. Claims have also been made of regression of early symptoms in response to the administration of heparin split products and of hyperbaric oxygen, but most have not been confirmed.
Among acute physical injuries to the spinal cord, those caused by electric currents and lightning, despite their rarity, are of great interest because they produce unusual clinical syndromes. Electrical forces can also injure the brain and peripheral nerves. These effects are noted only briefly here, because they are infrequent. It is the spinal cord that is most consistently and severely damaged.
In the United States, inadvertent contact with an electric current causes approximately 1,000 deaths annually and many more nonfatal but serious injuries. About one-third of the fatal accidents result from contact with household currents.
The factor that governs the damage to the nervous system is the amount of current, or amperage, with which the victim has contact, not simply the voltage, as is generally believed. In any particular case, the duration of contact with the current and the resistance offered by the skin to current (greatly reduced if the skin is moist or a body part is immersed in water) are of importance. The physics of electrical injuries is much more complex than these brief remarks indicate (for a full discussion, see the reviews by Panse and by Winkelman).
Any part of the peripheral or central nervous system may be injured by electric currents and lightning. The effects may be immediate, which is understandable, but of greater interest are the instances of neurologic damage that occur after a delay of 1 day to 6 weeks (1 week on average) and a rarer syndrome of anterior horn cell damage that arises after many years. The immediate effects are the result of direct heating of the nervous tissue, but the pathogenesis of the delayed effects is not well understood. They have been attributed to vascular occlusive changes induced by the electric current, a mechanism proposed to underlie the similar delayed effects of radiation therapy (see earlier). However, the latent period is measured in many months or a few years rather than in days and the course is more often progressive than self-limited. Moreover, the few postmortem studies of myelopathy as a consequence of electrical injury have disclosed a widespread demyelination of long tracts, to the point of tissue necrosis in some segments, and relative sparing of the gray matter, but no abnormalities of the blood vessels. There may also be spinal fracture from the vigorous muscle contraction.
The extraordinary syndrome of focal muscular atrophy occurring with a delay of weeks to years after an electric shock has been described by Panse under the title spinal atrophic paralysis. It occurs when the path of the current, usually of low voltage, is from arm to arm (across the cervical cord) or from an arm to leg. When the head is one of the contact points, the patient becomes unconscious or suffers tinnitus, deafness, or headache for a short period following the injury. Pain and paresthesias occur immediately in the involved limb but these symptoms are transient. Mild weakness, also unilateral, is immediate, followed in several weeks or months by muscle wasting, most often taking the form of segmental muscular atrophy. The syndrome simulates a regional form of amyotrophic lateral sclerosis (ALS) or transverse myelopathy (most patients have some degree of weakness and spasticity of the legs). However, we have encountered cases of asymmetric and profound atrophic weakness of the arms that began almost two decades after the shock and progressed over many years without long tract signs, both with a previously presumed diagnosis of amyotrophic lateral sclerosis. In contrast to injuries caused by high current, which affects mainly the spinal white matter (see earlier), it is the gray matter that is injured in cases of spinal atrophic paralysis, at least as judged from the clinical effects.
In a small number of surviving patients, after an asymptomatic interval of days to months, there has been an apoplectic onset of hemiplegia with or without aphasia or a striatal or brainstem syndrome, presumably because of thrombotic occlusion of cerebral vessels with infarction of tissue, but this condition has not been well studied.
The separate issue of the relationship of electrical shock exposure and the later development of typical ALS is quite controversial. Most series are hampered by retrospective acquisition of data about the shock. Although we have encountered a few remarkable instances of this association, including two who developed severe amyotrophy of the limb that was in contact with the electrical source many years before, a relationship to typical motor neuron disease has been considered coincidental.
The factors involved in injuries from lightning are less well defined than those from electric currents, but the effects are much the same. Direct strikes are often fatal; nearby strikes produce neurologic damage as described below. Topographic prominences such as trees, hills, and towers are struck preferentially, so these should be avoided; a person caught in the open should curl up on the ground, lying on one side with legs close together.
Arborescent red lines or burns on the skin indicate the point of contact of the energy generated by direct or nearby lightning. The path through the body can be approximately deduced from the clinical sequelae. Death is a result of ventricular fibrillation or of the effects of intense desiccating heat on the brain. Lightning that strikes the head is particularly dangerous, proving fatal in 30 percent of cases. Most persons struck by lightning are initially unconscious, irrespective of where they are struck. In those who survive, consciousness is usually regained rapidly and completely. Rarely, unconsciousness or an agitated-confusional state may persist for a week or two. Persistent seizures are surprisingly rare.
There is usually a disturbance of sensorimotor function of a limb or all the limbs, which may be pale and cold or cyanotic. As a rule, these signs are also evanescent, but in some instances they persist, or an atrophic paralysis of a limb or part of a limb makes its appearance after a symptom-free interval of several months as in the case of electrical injury.
A severe, predominantly motor polyneuropathy has been reported to appear after a variable interval and, while it bears similarities to the motor neuron disorder ostensibly associated with electrical injury discussed in the section above, there is a more persuasive relationship to the less common event of lightning. There are also a few cases on record of recovery from generalized polyneuropathy after lightning injury, but our experience with one case was of profound generalized axonal damage with little recovery (see Chap. 46).
This subject is introduced here with the other forms of spinal cord injury for want of a better way to categorize it. A transient and often asymmetric paraparesis is known to occur following prolonged spinal anesthesia but this is probably the result of a temporary effect of the injected agents on the cauda equina roots (see Chap. 46). A more serious and permanent injury has been caused by inadvertent injection of anesthetic directly into the conus medullaris (see Hamandi et al; Wilkinson et al). The patient reports leg weakness and numbness on one side immediately with the injection or upon awakening if sedation has been used. The MRI reveals an eccentrically placed traumatic lesion within the caudal spinal cord. Although this complication is rare, it has occurred even when experienced anesthesiologists perform the procedure; misidentification of the L3-L4 spinal interspace has been cited as the problem. Flat-tipped needles are as likely to cause injury to the conus as are ones with sharp beveled tips. Arachnoiditis from irritative agents, no longer used to any great extent, in the past caused a myelopathy (see Chap. 11).
Myelitis (Inflammatory Myelopathies)
In the nineteenth century, almost every disease of the spinal cord was labeled myelitis. Morton Prince, writing in Dercum’s Textbook of Nervous Diseases in 1895, referred to traumatic myelitis, compressive myelitis, and so on, obviously giving a rather imprecise meaning to the term. Gradually, knowledge of neuropathology advanced, and one disease after another was removed from this category until only the verifiably inflammatory ones remained.
The spinal cord is known to be the locus of a limited number of infective and noninfective inflammatory processes, some causing selective destruction of neurons, others affecting primarily white matter (tracts), and yet another group involving the meninges and white matter or leading to a necrosis of both gray and white matter. Other special terms, qualifying myelitis, are used to indicate more precisely the distribution of the process: if confined to gray matter, the proper expression is poliomyelitis; if to white matter, leukomyelitis. If approximately the whole cross-sectional area of the cord is involved at one or more levels, the process is said to be a transverse myelitis (although the term is still used more broadly for many myelitides); if the lesions are multiple and widespread over a long vertical extent, the modifying adjectives diffuse or disseminated are used and recently longitudinally extensive myelopathy has been introduced to denote a special form of necrotic myelopathy that is associated in most cases with particular circulating autoantibodies (see Chap. 36). The term meningomyelitis refers to combined inflammation of meninges and spinal cord and meningoradiculitis to combined meningeal and root involvement. An inflammatory process limited to the spinal dura is called pachymeningitis, and if infected material collects in the epidural or subdural space, it is called epidural or subdural spinal abscess or granuloma, as the case may be. The adjectives acute, subacute, and chronic denote the tempo of evolution of myelitic symptoms—namely, more or less within days, 2 to 6 weeks, or more than 6 weeks, respectively. The main causes of myelitis are listed below.
Viral myelitis (Chap. 33)
Enteroviruses (groups A and B Coxsackievirus, poliomyelitis, others)
Herpes zoster
Myelitis of AIDS
Epstein-Barr virus (EBV), cytomegalovirus (CMV), herpes simplex
Rabies
Arboviruses-Flaviviruses (Japanese, West Nile, etc.)
HTLV-I (human T-cell lymphotropic virus type I; tropical spastic paraparesis)
Myelitis secondary to bacterial, fungal, parasitic, and primary granulomatous diseases of the meninges and spinal cord (Chap. 32)
Mycoplasma pneumoniae
Lyme disease
Pyogenic myelitis
Acute epidural abscess and granuloma
Abscess of spinal cord
Tuberculous myelitis (Chap. 32)
Pott disease of the spine with secondary cord compression
Tuberculous meningomyelitis
Tuberculoma of spinal cord
Parasitic and fungal infections producing epidural granuloma, localized meningitis, or meningomyelitis and abscess, especially certain forms of schistosomiasis (Chap. 32)
Syphilitic myelitis (Chap. 32)
Chronic meningoradiculitis (tabes dorsalis)
Chronic meningomyelitis
Meningovascular syphilis
Gummatous meningitis including chronic spinal pachymeningitis
Sarcoid myelitis (Chap. 32)
Myelitis of noninfectious inflammatory type (Chap. 36)
Postinfectious and postvaccinal myelitis
Acute and chronic relapsing or progressive multiple sclerosis (MS)
Subacute necrotizing myelitis, neuromyelitis optica (NMO, Devic disease; longitudinally extensive myelopathy) due to antibodies against aquaporin (Chap. 36)
Myelopathy with lupus or other forms of connective tissue disease and antiphospholipid antibody
Paraneoplastic myelopathy and poliomyelitis (Chap. 31)
From this outline it is evident that many different and totally unrelated diseases are under consideration and that a general description cannot possibly encompass such a diversity of processes. Overall, myelitis caused by multiple sclerosis and postinfectious processes are the most common causes in practice. This was the case in the series collected by de Seze and colleagues (2001a); Nowak and coworkers reported a similar distribution. Many of the myelitides are considered elsewhere in this volume in relation to the diseases of which they are a part. Here it is only necessary to comment on the principal categories and to describe a few of the common subtypes.
The enteroviruses, of which Coxsackie and poliomyelitis are examples, herpes zoster, arboviruses such as West Nile and the equine encephalitic viruses, and HIV are the important members of this category. The enteroviruses in particular have an affinity for neurons of the anterior horns of the spinal cord and the motor nuclei of the brainstem (i.e., they are neuronotropic and cause a disease that can be generically termed poliomyelitis), and herpes zoster virus has a clear affinity for the dorsal root ganglia; hence the disturbances of function are in terms of motor and sensory neurons, respectively, not of spinal tracts. We have cared for several patients who have had destruction of anterior horn cells as a consequence of an enterovirus other than poliomyelitis virus (see further on). West Nile virus shows the same proclivity to damage anterior horn cells. The onset of these conditions is acute and takes the form of a febrile meningomyelitis. Although there are fever, systemic symptoms, and sometimes, cutaneous features (in the case of zoster), it is the nervous system disorder that is most significant. The patient suffers the immediate effects of nerve cell destruction, and some degree of improvement nearly always follows as some neurons recover. Later in life, possibly as the neuronal loss of aging reduces the number of anterior horns, there may be an apparent increased loss of strength in muscles originally weakened by poliomyelitis (“postpolio” syndrome).
Relatively infrequent examples of a more or less transverse myelitis caused by herpes simplex virus (HSV types 1 and 2), varicella-zoster virus (VZV), CMV, EBV, any of the hepatitis viruses, and SV70 virus (causing epidemic conjunctivitis) have been reported, some in patients with immunodeficiency states, mainly AIDS. The situation is complex clinically, as most of these agents may also elicit a postinfectious variety of myelitis, described further on in this chapter and in Chaps. 33 and 36. HSV type 2 and CMV infections may also produce an acute lumbosacral radiculitis with urinary retention (Elsberg syndrome). A few cases of zoster myelitis have shown evidence of extensive inflammatory necrosis of the spinal cord with involvement of sensory and motor tracts, causing acute paraplegic and tetraplegic transverse syndromes. Pleocytosis in the cerebrospinal fluid and isolation of the viral DNA from the cerebrospinal fluid confirm the diagnosis of a primary viral infection as discussed in Chap. 33.
There are other rare forms of poliomyelitic reactions of unknown, possibly viral etiology. One such condition presents as an acute febrile or afebrile meningomyelitis and leaves all the limbs paralyzed and flaccid, sparing the brainstem and affecting the diaphragm to a variable extent. Several such patients have harbored a cancer or Hodgkin disease, and the pathology was more typical of a poliomyelitic viral infection rather than of the usual paraneoplastic syndromes (Chap. 31). Involvement of the white matter with sensory and motor paralysis below the level of a lesion has also been reported in so-called “dumb” rabies (in contrast to the usual form of “mad” or “furious” rabies encephalitis), and in an infection transmitted by the bite of a monkey, called the B virus. These are decidedly rare. More common are the viral myelopathy of HIV-AIDS and of HTLV-I infection. With these exceptions, one may say that myelitis that expresses itself mainly by dysfunction of motor and sensory tracts will usually prove not to be viral in origin but rather to one of the disease processes in category III (noninfectious, inflammatory) of the preceding classification, for example, multiple sclerosis. The unique myelopathies of HIV and of human HTLV infections are described below.
As the neurology of AIDS has been elucidated, the clinical and pathologic characteristics of a viral myelopathy have been studied in detail. The frequency of this condition is impressive—it was present in 20 of 89 successive cases of AIDS on whom a postmortem examination was performed by Petito and colleagues. Often, the clinical symptoms and signs of spinal cord disease are obscured by a neuropathy or one or more of the cerebral disorders that complicate AIDS either because of HIV or due to an opportunistic infection (CMV, toxoplasmosis, progressive multifocal leukoencephalopathy [PML]). In 5 cases of severe vacuolar myelopathy in the aforementioned series, there was leg or leg and arm weakness, often asymmetrical and developing over a period of weeks, to which the signs of sensory tract involvement and sphincteric disorder were added. A sensory ataxia has also been a common early feature in our experience. The CSF shows a small number of lymphocytes, a slight elevation of protein, and, occasionally, bizarre giant cells.
The white matter of the spinal cord is vacuolated, by which is meant a ballooning within myelin sheaths of the long tracts. The changes are most severe in thoracic segments with the posterior and lateral columns are affected diffusely. Axons are involved to a lesser degree, and lipid-laden macrophages are present in abundance. Similar vacuolar lesions may be seen in the brain in some cases. The lesions in the spinal cord resemble those of subacute combined degeneration but levels of vitamin B12 and folic acid are normal. (A similar lesion was found in one of our patients with myelopathy from chronic lupus erythematosus.)
The antiretroviral drugs that slow the progress of AIDS, with the exception of a few cases, seem to have little effect on the myelopathy and one can only resort to symptomatic treatment of spasticity.
This disease was brought to the attention of neurologists 50 years ago through the observations and writings of Cruickshank. However, it is only more recently that a chronic infective-inflammatory disease of the spinal cord caused by the retrovirus HTLV-I has been discovered and its connection to what had been called tropical myelitis, appreciated. The implications of this discovery are potentially broad and extend even to the demyelinative and possibly the degenerative diseases.
Spinal cord disease of this type has been reported from the Caribbean islands, southeastern United States, southern Japan, South America, and Africa. The clinical picture is one of a slowly progressive paraparesis with increased tendon reflexes and Babinski signs; disorder of sphincteric control is usually an early feature but symmetric paresthesias, reduced vibratory and position senses, and ataxia follow over several months or years. A few patients have had an associated polyneuropathy, as in Cruickshank’s early cases. The upper extremities are usually spared (except for lively tendon reflexes), as are cerebral and brainstem functions.
The CSF contains small numbers of T-lymphocytes (10 to 50/mm3), normal concentrations of protein and glucose, and an increased content of immunoglobulin (Ig) G with antibodies to HTLV-I. The diagnosis is confirmed by the detection in the serum of the antibodies to the virus. Thinness of the spinal cord is evident on MRI and subcortical cerebral white matter lesions may be seen as well. Neuropathologic study has documented an inflammatory myelitis with focal spongiform, demyelinative, and necrotic lesions, perivascular and meningeal infiltrates of inflammatory cells, and focal destruction of gray matter. The posterior columns and corticospinal tracts are the main sites of disease, most evident in the thoracic cord.
Because of slow evolution, the clinical picture can easily be confused with that of progressive spastic paraplegia of the heredofamilial variety, sporadic motor neuron disease, or the chronic phase of multiple sclerosis. There are also similarities with the AIDS myelopathy described earlier, but the other features of HIV infection are absent. There are reports of improvement with intravenous administration of gamma globulin but these have not been consistent and had no effect in two of our patients.
With few exceptions, this class of spinal cord disease seldom offers difficulty in diagnosis. The CSF usually holds the clue to causation. In most cases, the inflammatory reaction in the meninges is only one manifestation of a generalized (systemic) disease process. The spinal lesion may involve primarily the pia-arachnoid (leptomeningitis), the dura (pachymeningitis), or the epidural space, e.g., taking the form of a compressive abscess or granuloma; or it may reside in the adjacent spinal bones. In some acute forms both the spinal cord and meninges are simultaneously affected, or the cord lesions may predominate. Chronic spinal meningitis may involve the pial arteries or veins; and as the inflamed vessels become thrombosed, infarction (myelomalacia) of the spinal cord results. Chronic meningeal inflammation may provoke a progressive constrictive pial fibrosis (spinal arachnoiditis) that virtually strangulates the spinal cord. In certain cases, spinal roots become progressively damaged, especially the lumbosacral ones, which have a long meningeal exposure. Posterior roots, which enter the subarachnoid space near arachnoidal villi (where CSF is resorbed) tend to suffer greater injury than anterior ones (as happens in tabes dorsalis). Interestingly, there are cases of chronic cerebrospinal meningitis that remain entirely without symptoms until the spinal cord or roots become involved.
The infrequent but curious bacterial myelitis caused, or in some way precipitated, by the atypical pneumonia agent M. pneumoniae has come to be viewed as a postinfectious immune disease, as discussed in Chap. 32. However, portions of the DNA from this organism have been found in the spinal fluid early in the course of illness in some cases, suggesting the possibility of a direct bacterial infection of the spinal cord. It is not known whether antibiotic treatment alters the course of the illness.
Syphilitic myelitis is also discussed in Chap. 32. Bacterial abscess of the spinal cord is rare, especially in comparison to epidural spinal abscess, and it is recognized by MRI. At times, it stands as a single pyogenic metastasis from a distant infection and subsequent bacteremia, but more often there has been spread from a contiguous infected surgical site or a fistulous connection with a superficial paraspinal abscess. Vertebral osteomyelitis is addressed further on in relation to epidural abscess.
Sarcoid granulomas may occur as one or more intramedullary spinal cord masses, as in the cases reported by Levivier and colleagues. In our experience, the granulomatous lesion, which may be focal or multifocal, simulates demyelinative disease with respect to its tendency to relapse and remit and in its response to corticosteroids. An asymmetrical ascending paraparesis and bladder disturbance have been the main features in our patients. Usually there is evidence of systemic sarcoidosis and the CSF is abnormal (increase in cells and protein; glucose usually normal), but we have encountered a few instances of sarcoid restricted to the spinal cord before it was evident in the mediastinum (i.e., thoracic CT failing to demonstrate hilar adenopathy or diffuse parenchymal lung disease). Elevation of the spinal fluid IgG concentration and oligoclonal bands may be found, but they are not consistent features; often there are activated histiocytes in the CSF.
The use of angiotensin-converting enzyme levels in the CSF to distinguish sarcoidosis from multiple sclerosis suffers from the lack of normative values for this test, but it is reported in some small series to be elevated in two-thirds of patients. The MRI is abnormal and the conus or other portions of the cord reveal intramedullary lesions. The most characteristic finding, however, is a multifocal-subpial nodular enhancement of the meninges adjacent to a lesion within the cord or nerve roots—a picture that resembles neoplastic meningeal infiltration. The diagnosis can be confirmed by mediastinal lymph node biopsy or by the far less desirable method of biopsy of the spinal meninges, spinal roots and affected subpial cord.
On occasion, a number of other rare granulomatous conditions cause an intrinsic or, more often, extrinsic compressive myelopathy; these, include brucellosis, xanthogranulomatosis, and eosinophilic granuloma. The diagnosis may be suspected if the systemic disease is apparent at the time but usually, only the histology of a surgical specimen reveals the underlying process.
This condition is worthy of emphasis because the diagnosis is often missed or mistaken for another disease, sometimes with disastrous results. Children or adults may be affected. Infection of the epidural space has a wide variety of sources. Staphylococcus aureus is the most frequent etiologic agent, followed in frequency by streptococci, gram-negative bacilli, and anaerobic organisms. An injury to the back, often trivial at the time, furunculosis or other skin or wound infection, or a bacteremia may permit seeding of the spinal epidural space or of a vertebral body. This gives rise to osteomyelitis with extension of the purulent process to the epidural space. Occasionally, the spread is from an infected disc. Another source is bacteremia in a drug addict following the use of nonsterile needles or the injection of contaminated drugs.
Organisms may be introduced into the epidural space during spinal surgery or rarely via a lumbar puncture needle during epidural or spinal anesthesia or from epidural injections of steroid or other therapeutic agents; the localization is then over the lumbar and sacral roots. In these cases of cauda equina epidural abscess, back pain may be severe but neurologic symptomatology is minimal unless the infection extends upward to the upper lumbar and thoracic segments of the spinal cord. It must be acknowledged that some, even fulminant, cases have no clear source in the body for the bacterial abscess.
At first, the purulent process in the cervical or thoracic region is accompanied only by low-grade fever and aching local back pain, usually intense, in most cases followed within a day or several days by radicular pain. Headache and nuchal rigidity are sometimes present; more often there are just persistent pain and a disinclination to move the back. After several days, there may be a rapidly progressive paraparesis associated with sensory loss in the lower parts of the body and sphincteric paralysis. Percussion of the spine elicits considerable tenderness over the site of the infection. Examination discloses all the signs of a complete or partial transverse cord lesion, including at times the elements of spinal shock if paralysis has evolved rapidly.
The diagnosis can usually be ascertained from MRI (Fig. 44-2) but care must be taken to obtain images from levels rostral and caudal enough to detect the infected collection. There may be enhancement of the margins of the purulent collection after several days.
If a diagnostic spinal puncture is performed, the CSF usually contains white cells but of surprisingly small number (fewer than 100/mm3), both polymorphonuclear leukocytes and lymphocytes, unless of course, the needle penetrates the abscess, in which case pus is obtained. The CSF protein content is high, (100 to 400 mg/100 mL or more) but the glucose is normal. Elevation of the sedimentation rate, C-reactive protein, and peripheral neutrophilic leukocytosis are additional indicators to the diagnosis. The last of these tests is abnormal in two-thirds of cases. Blood cultures demonstrate the organism in a similar proportion. Cultures from the CSF are infrequently positive. The decades old series reported by Baker and colleagues is still a valuable reference, as is the more recent discussion by Darouiche. The differential diagnosis includes other forms of spinal cord compression and, in cases with areflexic spinal shock, tetraparesis and respiratory failure, Guillain-Barré syndrome.
The foregoing clinical findings call for MRI to be performed relatively quickly or CT myelography if MRI is not possible, to demonstrate the mass of the abscess and to determine its level. If not treated surgically by laminectomy and drainage before the onset of paralysis, the spinal cord lesion, which is probably partly a result of venous ischemia, becomes more or less irreversible. Broad-spectrum antibiotics in large doses must be given initially and the choice of treatment is then refined based on cultures from the abscess or the blood, or on a presumed source of bacteria, usually found to be staphylococcus.
When osteomyelitis of a vertebral body is the primary abnormality, the epidural extension may implicate only a few spinal sensory and motor roots, leaving long tracts and other intramedullary structures intact. In some cases with cervical epidural abscesses, stiff neck, fever, and deltoid-biceps weakness are the main neurologic abnormalities. Having emphasized the urgency of treatment, there are instances of small epidural abscesses that do not compress the cord and are limited to one or at most two levels for which we have avoided surgery by administering antibiotics alone. Also, lumbar epidural abscess and cauda equina compression without neurologic signs may be, in some cases, treated solely with antibiotics, although many surgeons favor drainage, which must be undertaken in any case, if osteomyelitis develops. Antibiotics are continued for several weeks, and the patient should be examined at regular intervals and have sequential MRI scans of the affected region.
Even after apparently successful drainage and antibiotic treatment of an epidural abscess, there may be a slowly progressive and then static syndrome of partial spinal cord compression. This is the result of formation of a fibrous and granulomatous reaction at the operative site. Distinguishing this sterile inflammatory mass from residual epidural abscess is quite difficult, even with enhanced MRI, but persistent fever, leukocytosis, and an elevated sedimentation rate, C-reactive and peripheral white blood cell count suggest that surgical drainage of the abscess was incomplete.
Spinal subdural abscess due to bacterial infections also occur and, clinically, are virtually indistinguishable from epidural ones on clinical grounds. The MRI will usually clarify the situation but a clue is provided by the CT myelogram, in which the subdural lesion has a less sharp margin and usually, a greater vertical extent. The epidural and subdural infections, if they smolder owing to delayed diagnosis or inadequate therapy, may also evolve into a local chronic adhesive meningomyelitis.
Subacute pyogenic infections and granulomatous infections (tuberculous, fungal) may also arise in the spinal epidural space, as noted below.
Purulent collections within the substance of the cord was first described by Hart in 1830 and, although it is rare, 73 cases had been reported by 1994 (Candon and Frerebeau). In some instances, the patient was known to have had systemic bacterial infection, septicemia, or endocarditis; in others, there was a contiguous abscess in the skin or subcutaneous tissues with a fistula to the spinal cord through an intervertebral foramen. Spinal cord abscess is a rare complication of spinal dysraphism or of a developmentally open dorsal fistulous tract. The symptoms are indistinguishable from those of epidural abscess, namely, spinal and radicular pain followed by sensory and motor paralysis; the CSF findings are also the same. Woltman and Adson described a patient in whom surgical drainage of an encapsulated intramedullary abscess led to recovery, and Morrison and associates reported a similar case caused by Listeria monocytogenes, which was successfully drained and the meningeal infection suppressed by ampicillin and chloramphenicol. There was no way to be certain of the diagnosis prior to the availability of MRI.
This process is presented in juxtaposition to epidural abscess, with which it is closely aligned. As with other forms of osteomyelitis, vertebral infection is typically due to hematogenous implantation of bacteria during episodes of bacteremia or, it is associated with the exogenous introduction of bacteria during spinal surgery, particularly if catheters or other devices, including for spinal stabilization, are incorporated. In the case of postsurgical infection, coagulase-negative staphylococci or propionibacterium are almost always implicated, whereas with bacteremia, a number of low-virulence organisms including staphylococcus are found and multiple organisms may be involved. The source of bacteremia may be urinary infection, endocarditis, or intravenous drug abuse but many affected individuals also have diabetes, are immunosuppressed, or receiving dialysis for renal failure. In the group of immunocompromised patients, unusual or endemic organisms such as Brucella may be found. However, in almost half of patients who have not had surgery, no source is identified. Approximately one-fifth of cases have an associated epidural abscess, as discussed above. This is often indicated by an increase in local back pain or extremely severe pain from the onset.
The lumbar spine is the region most affected. The typical presentation is relatively nondescript with back pain, elevated white blood cell count and see-reactive protein level. Fever, however, is inconsistent. Several types of imaging studies may be used to demonstrate the infection, however, MRI more dependably than CT shows edema within the bone marrow and, if there is destruction of the disc adjacent to an effective vertebral body, infection is almost certain. Technetium bone scans were popular for the demonstration of osteomyelitis in general but the findings may be nonspecific. A well-known adage is that neoplasms affecting the vertebral body do not cross the disc space.
A point of contention has been the need for biopsy of the affected bone when blood cultures are negative and no obvious source of infection in the body can be found. This procedure is generally suggested although extension of the infection from the vertebral body to the paravertebral or epidural spaces may also be performed under CT guidance.
Initiating therapy with oral fluoroquinolones, with or without rifampin, has been suggested as a broad approach while the specific infecting bacteria are identified. Therapy is generally continued for at least 4 to 6 weeks, if not longer but no clear guidance is available on the appropriate duration. Surgical removal of infected bone is generally not undertaken unless the osteomyelitis is the result of implanted hardware during previous spinal surgery. Almost invariably, this hardware must be removed. Surveillance for persistent infection after treatment is probably appropriate but the MRI has not proven useful for this purpose. Inflammatory markers in the blood are apparently more dependable. A thorough review of this subject can be found in the clinical practice article by Zimmereli.
Tuberculous osteomyelitis of the spine with kyphosis (Pott disease) is well known in regions of endemic tuberculosis. Children and young adults are most often affected. The osteomyelitis is the result of reactivation of tuberculosis at a site previously established by hematogenous spread. An infectious endarteritis causes bone necrosis and collapse of a thoracic or upper lumbar (less often cervical) vertebral body resulting in a characteristic angulated kyphotic deformity (Fig. 44-3); any degree of additional rotary instability allows the emergence of a gibbus deformity. Most patients have some active tuberculous infection as evidenced by fever, night sweats, and other constitutional symptoms; the sedimentation rate is invariably elevated but the degree may be slight. A compressive myelopathy occurs in some cases as a result of the spinal deformity, but it is infrequent and an epidural tuberculous abscess is a more common cause of cord compression (see below). What is surprising to us about Pott disease is the excellent result that may be obtained by external stabilization of the spine and long-term antituberculous medication. A recent young patient of ours was saved from an operation by the intercession by telephone of his father, a physician from India. Although there is some controversy regarding spinal surgery, it is certainly required in the presence of severe deformities or a compressive myelopathy as noted in Chap. 32.
Solitary tuberculoma of the spinal cord as part of a generalized infection is a rarity. More often, pus or caseous granulation tissue extrudes from an infected vertebra and gives rise to an epidural compression of the cord (Pott paraplegia, as distinct from Pott disease). Occasionally tuberculous meningitis may result in pial arteritis and spinal cord infarction. The paraplegia may appear before the tuberculous meningitis is diagnosed. All these forms of tuberculosis are infrequent in the United States and Western Europe, but we see a new case every several years in a patient who had spent his earlier life in India or Africa. Additional comments can be found in Chap. 32.
A wide variety of fungal and parasitic agents may involve the spinal meninges. Such infections are rare, and some do not occur at all in the United States or are limited to certain geographic areas, particularly among immigrant populations. Actinomyces, Blastomyces, Coccidioides, and Aspergillus may invade the spinal epidural space via intervertebral foramina or by extension from a vertebral osteomyelitic focus. Cryptococcus, which causes meningoencephalitis and, rarely, a cerebral granuloma, in our experience, seldom causes spinal lesions. Hematogenous metastases to the spinal cord or meninges may occur in both blastomycosis and coccidioidomycosis. Occasionally an echinococcal infection of the posterior mediastinum may extend to the spinal canal (epidural space) via intervertebral foramina and compress the spinal cord.
Schistosomiasis (bilharziasis) is a recognized cause of myelitis in the Asia, Africa, and South America. The spinal cord is a target for all three common forms of Schistosoma: S. haematobium, S. japonicum, and S. mansoni, but most particularly the last of these (see “Schistosomiasis” in Chap. 32). The schistosomal ova evoke an intense granulomatous myelomeningoradiculitis. The lesions are destructive of gray and white matter, with ova in arteries and veins leading to vascular obstruction and ischemia (Scrimgeour and Gajdusek). Less often, a localized granuloma gives rise to a cord syndrome and, rarely, the disease takes the form of an acute transverse myelitis with massive necrosis of cord tissue (Queiroz et al). A pruritic “swimmer’s itch” at the site of entry of the parasite is reported by many patients in the days prior to the myelopathy. In the often cited review by Scrimgeour and Gajdusek, the latency between exposure and symptoms was 38 days to several years. We have cared for several patients over the years in whom the spinal cord in the low thoracic and lumbar region was infected approximately 3 weeks after they swam in contaminated water during an east African vacation and then returned home to the United States The CSF showed only a slight elevation of protein, but in almost all cases there is a pronounced pleocytosis ranging from 5 to 500 lymphocytes/mm3 and the glucose is normal or minimally reduced. Systemic and CSF eosinophilia are variable so are not dependable for diagnosis. The diagnosis is confirmed by the finding of elevated titers of antibody directed against the schistosome in the CSF or blood. There are usually oligoclonal bands of IgG in the CSF as well. The parasite can sometimes be found in biopsies of the rectosigmoid mucosa. The administration of praziquantel arrested the course of the illness, but all but one of our patients was left disabled.
Myelitis of Noninfectious Inflammatory Type (Multiple Sclerosis and Acute and Subacute Transverse Myelitis) (See Chap. 36)
The spinal cord disorders that make up this category take the form of a leukomyelitis based on either demyelination or necrosis of portions of the spinal cord. The critical factor in their pathogenesis appears to be a disordered immune response, in some cases, as a response to an infection, and in others such as multiple sclerosis an idiopathic immune disorder. Varied clinical syndromes are produced, and the basic disease is classified in textbooks under headings such as acute transverse myelitis, postinfectious myelitis, postvaccinal myelitis, acute MS, neuromyelitis optica, and necrotizing myelitis. While each of these conditions may affect other parts of the nervous system (most often the optic nerves and brain), often the only manifestations are spinal. The aforementioned myelopathies are characterized by various degrees of inflammatory destruction, usually with lymphocytes congregating around venules in the cord, but they are sufficiently distinct to justify their separate classification. Nonetheless, transitional cases sharing the clinical and pathologic attributes of more than one disease are encountered in any large clinical practice and pathologic collection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree