Diseases of the Cranial Nerves: Introduction
The cranial nerves are susceptible to a number of special diseases, some of which do not affect the spinal peripheral nerves. For this reason alone they are considered separately. Certain of the cranial nerves and their disorders have already been discussed: namely, disorders of olfaction in Chap. 12; of vision and extraocular muscles in Chaps. 13 and 14; of cochlear and vestibular function in Chap. 15; and craniofacial pain in Chap. 10. There remain to be described the disorders of the facial (VII) nerve and of the lower cranial nerves (IX to XII), as well as certain diseases that affect the trigeminal (V) nerve. These are considered here.
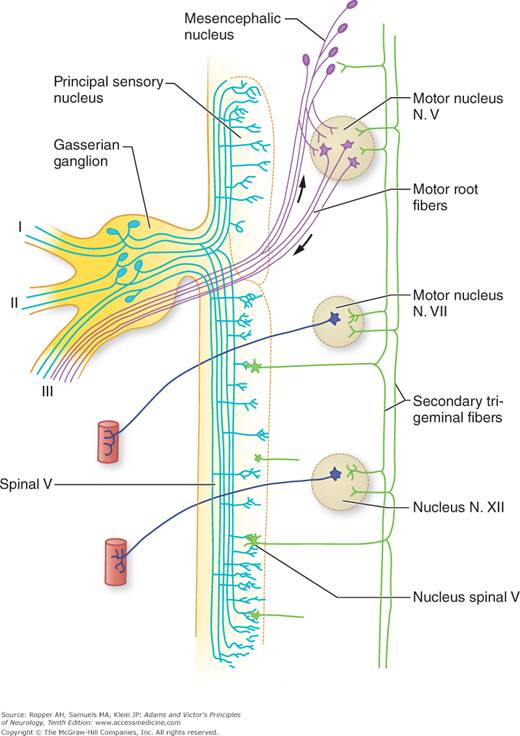
The fifth nerve (Fig. 47-1) is a mixed sensory and motor nerve. It conducts sensory impulses from the greater part of the face and head; from the mucous membranes of the nose, mouth, and paranasal sinuses; and from the cornea and conjunctiva. It also provides the sensory innervation of the dura in the anterior and middle cranial fossae. The cell bodies of the sensory part of the nerve lie in the gasserian, or semilunar, ganglion. This, the largest sensory ganglion in humans, lies in the inferomedial part of the middle cranial fossa in a recess called Meckel’s cave. The central axons of the ganglion cells form the sensory root of the nerve. These fibers, on entering the lateral mid pons, divide into short ascending and long descending branches. The former are concerned mainly with tactile and light pressure sensation and synapse with second-order neurons in the principal sensory nucleus. Proprioceptive afferents from facial muscles and the masseter also ascend to terminate in the mesencephalic nucleus. The fibers that mediate pain and temperature sensation do not end in these nuclei but form long descending branches of the spinal trigeminal tract. This pathway, which contains both facilitatory and inhibitory fibers, together with its adjacent nucleus, extends from the junction of the pons and medulla to the uppermost segments (C2 or C3) of the spinal cord (as evidenced by the relief of facial pain after medullary trigeminal tractotomy).
Figure 47-1.
Scheme of the trigeminal nuclei and some of the trigeminal reflex arcs. I, ophthalmic division; II, maxillary division; III, mandibular division. (Originally from Ramon y Cajal S: La Textura del Sistema Nervista del Hombre y los Vertebrados, Madrid, Moya, as adapted from Carpenter MB, Sutin J: Human Neuroanatomy, 8th ed. Baltimore, Williams & Wilkins, 1982, by permission.)
The spinal trigeminal nucleus in the upper cervical cord is a continuation of the spinal tract of Lissauer and substantia gelatinosa; the main trigeminal sensory nucleus in the pons and medulla is a continuation of the nucleus of the medial lemniscus. From all parts of the principal sensory and spinal trigeminal nuclei, second-order fibers cross to the opposite side and ascend to the thalamus. They come to lie in the most medial part of the spinothalamic tract and lateral part of the medial lemniscus. These systems of fibers are called the trigeminothalamic tract. In addition, the secondary trigeminal neurons project to the facial and hypoglossal nuclei bilaterally, the salivatory nuclei, the cuneate nuclei of the upper cervical segments, and other cranial nerve nuclei. The principal sensory and spinal trigeminal nuclei receive fibers from the reticular formation, the thalamus, the nucleus tractus solitarius, and the somatosensory cortex.
The peripheral branches of the gasserian ganglion form the three sensory divisions of the nerve. The first (ophthalmic) division passes through the cavernous sinus and superior orbital fissure; the second (maxillary) division also passes through the cavernous sinus and leaves the middle fossa through the foramen rotundum; and the third (mandibular), does not traverse the cavernous sinus and instead exits Meckel’s cave inferiorly through the foramen ovale.
The motor portion of the fifth nerve, which supplies the masseter and pterygoid muscles, has its origin in the trigeminal motor nucleus in the mid pons; the exiting fibers pass underneath (but not through) the gasserian ganglion and become incorporated into the mandibular nerve. The masseter and pterygoid muscles are used in chewing and are implicated in a number of brainstem reflexes, the best known of which is the jaw jerk. Tapping the chin with the jaw muscles relaxed stimulates proprioceptive afferents that terminate in the mesencephalic nucleus of the midbrain, which sends collaterals to the motor nucleus of the fifth nerve and causes the masseters to contract. This reflex is enhanced in spastic bulbar (pseudobulbar) palsy. Another pontine reflex that uses afferent trigeminal sensory nerves is the blink reflex. Tapping of the brow or bridge of the nose evokes bilateral blink through activation of the orbicularis oculi muscles (facial nerve efferents). Touching the eyelids and cornea (corneal reflex) does the same.
Because of their wide anatomic distribution, complete interruption of both the motor and sensory fibers of the trigeminal nerve is rarely observed. In contrast, partial dysfunction of the trigeminal nerve, particularly of the sensory part, is common, the main symptoms being facial numbness and pain. The various cranial nerve and brainstem syndromes in which the fifth nerve is involved are listed in Tables 47-1, 31-5, and 34-3, the last in relation to stroke syndromes of the brainstem that affect the nerve in its fascicular course or in its nucleus.
SITE | CRANIAL NERVES INVOLVED | EPONYMIC SYNDROME | USUAL CAUSE |
|---|---|---|---|
Sphenoidal fissure | III, IV, ophthalmic, V, VI | Foix | Invasive tumors of sphenoid bone, aneurysms |
Lateral wall of cavernous sinus | III, IV, ophthalmic (occasionally maxillary), V, VI | Tolosa-Hunt | Aneurysms or thrombosis of cavernous sinus; invasive tumors from sinuses and sella turcica; sometimes recurrent, benign granulomatous reactions, responsive to steroids |
Retrosphenoidal space fossa | II, III, IV, V, VI | Jaccoud | Large tumors of middle cranial |
Apex of petrous bone | V, VI | Gradenigo | Petrositis, tumors of petrous bone |
Internal auditory meatus | VII, VIII | Tumors of petrous bone (dermoids, etc.), vestibular schwannoma | |
Pontocerebellar angle | V, VII, VIII, and sometimes IX | Vestibular schwannomas, meningiomas | |
Jugular foramen | IX, X, XI | Vernet | Tumors (glomus jugulare), venous sinus thrombosis, and aneurysms |
Posterior laterocondylar space | IX, X, XI, XII | Collet-Sicard | Tumors of parotid gland, carotid body; secondary and lymph node tumors, tuberculous adenitis, carotid artery dissection |
Posterior retroparotid space | IX, X, XI, XII, and Horner syndrome | Villaret | Same as above, and granulomatous lesions (sarcoid, fungi) |
Posterior retroparotid space | X and XII, with or without XI | Tapia | Parotid and other tumors of, or injuries to, the high neck |
A variety of diseases may affect the peripheral branches of the trigeminal nerves, the gasserian ganglion, and the roots (sensory and motor). Hughes has summarized them and the main ones are described below. The role of the nerve in migraine is discussed in Chap. 10.
(See also “Trigeminal Neuralgia” in Chap. 10) The most frequent and at the same time the most elusive disease of the fifth nerve from the standpoint of its pathologic basis is trigeminal neuralgia (tic douloureux). This condition has been known since ancient times, having been described by Arateus in the first century A.D., by John Locke in 1677, by Nicolaus Andre in 1756, and by John Fothergill in 1776 (according to Katusic et al). The overall incidence rate for both sexes combined is 4.3 per 100,000 persons per year, but it is higher for women than for men (in a ratio of 3:2) and is much higher in the elderly. The mean age of onset is 52 to 58 years for the idiopathic form and 30 to 35 years for the symptomatic forms, the latter being caused by trauma or vascular, neoplastic, and demyelinative diseases. In the last decade it has become apparent, mainly from the work of Jannetta, that a proportion of cases is a result of compression and secondary demyelination of trigeminal nerve rootlets by small branches of the basilar artery (see Love and Coakham).
The paroxysmal nature of the facial pain, its unilaterality, the tendency to involve the second and third divisions of the trigeminal nerve, an intensity that makes the patient grimace or wince (tic), the presence of a trigger point on the face, the lack of demonstrable sensory or motor deficit, and its response in more than half of the cases to antiepileptic drugs are characteristic. The diagnosis of “idiopathic” trigeminal neuralgia and its differentiation from other forms of intermittent facial pain described below—as well as from cluster headache, dental neuralgia, temporomandibular joint pain, and atypical facial pain—is usually not difficult, especially if there is a trigger point and no demonstrable evidence of sensory or motor impairment. Furthermore, the vascular compressive form is difficult to diagnose without high-resolution neuroimaging or exposure at operation and most such cases are therefore characterized as idiopathic until revealed as vascular in causation.
In rare instances, trigeminal neuralgia is preceded or accompanied by hemifacial spasm, a combination that Cushing called tic convulsif. This may be indicative of a tumor (cholesteatoma), an aneurysmal dilatation of the basilar artery or one of its branches, or an arteriovenous malformation that compresses both the trigeminal and facial nerves. Trigeminal neuralgia and glossopharyngeal neuralgia (pain in the tonsillar region) may also be combined in these conditions.
Of the conditions that damage the branches of the trigeminal nerve, facial and cranial injuries, and fractures are probably the most common, but they do not usually come to the attention of neurologists. The most superficial branches of the nerve—the supratrochlear, supraorbital, and infraorbital—are the ones usually involved by trauma. The sensory loss is present from the time of the injury, and partial regeneration may be attended by constant pain.
Of the various inflammatory and infectious diseases that affect the trigeminal nerves or ganglia, herpes zoster ranks first. Persistent pain after herpetic infection of the fifth nerve is a serious problem, not responding well to any type of treatment. This subject is discussed in Chap. 10 with other forms of facial pain. Middle ear infections and osteomyelitis of the apex of the petrous bone may spread to the ganglion and root, also implicating the sixth cranial nerve (Gradenigo syndrome). HIV infection has not been clearly implicated in infection of the fifth nerve (as it has in the seventh nerve), but reactivation of latent herpes zoster is seen with AIDS.
The trigeminal root may be compressed or invaded by intracranial meningiomas, vestibular schwannomas, trigeminal schwannomas, cholesteatomas, and chordomas and by tortuous branches of the basilar artery. Sinus tumors and metastatic disease may also infiltrate the nerve, causing pain and a gradually progressive sensory loss. Demyelination at the trigeminal root entry point into the pons is another well-characterized cause in cases of multiple sclerosis (Fig. 47-2).
The ophthalmic division of the fifth nerve may be involved in the wall of the cavernous sinus in combination with the third, fourth, and sixth nerves by a variety of processes, including thrombosis of the cavernous sinus. Tumors of the sphenoid bone (myeloma, metastatic carcinoma, squamous cell carcinoma, and lymphoepithelioma of the nasopharynx) may involve branches of the trigeminal nerve at their foramina of entry or exit. An unusual perineural infiltration of superficial branches of the nerve by squamous cell skin cancers of the face is discussed further on under “Multiple Cranial-Nerve Palsies.” The mandibular division of the nerve may be compressed by the roots of an impacted third molar (wisdom) tooth. Well known to clinicians is a sign of numbness of the chin and lower lip from infiltration of the mental nerve as the first indication of metastatic carcinoma of the breast, prostate, or multiple myeloma. Massey and colleagues have described the details of 19 such cases of the “numb-chin” sign.
Neurologists also encounter instances of slowly evolving unilateral or bilateral trigeminal neuropathy in which sensory impairment is confined to the territory of the trigeminal nerve, sometimes associated with pain, paresthesias, or disturbances of taste. This type of loss of facial sensation can also occur as part of a widespread sensory neuropathy or ganglionopathy that occurs as a paraneoplastic effect of cancer (see Chap. 31) or with Sjögren disease.
As common is an association between isolated trigeminal neuropathy and immune-mediated connective tissue diseases. Of 22 such cases described by Lecky and colleagues, 9 had either scleroderma or mixed connective tissue disease, and a similar number had either organ- or nonorgan-specific serum autoantibodies. Several specific antibody tests are used to establish the diagnosis of scleroderma. The symptoms may involve the other side of the face years later. Hughes has also described cases of trigeminal neuropathy with scleroderma, lupus erythematosus, and Sjögren disease. We have seen several patients with Sjögren disease in whom the trigeminal neuropathy and the associated antibodies or inflammation of the minor salivary glands were evident well before the characteristic sicca syndrome or other systemic manifestations of the disease. The condition may remain troublesome for years. Pathologic data are limited but point to an inflammatory lesion of the trigeminal ganglion or sensory root. Stilbamidine and trichloroethylene are known to cause sensory loss, tingling, burning, and itching exclusively in the trigeminal sensory territory.
Spillane and Wells, many decades ago, discussed an isolated trigeminal neuropathy (it had been called Spillane’s trigeminal neuritis). Four of their 16 patients had an associated paranasal sinusitis, but subsequent reports have failed to substantiate a causal relationship between sinusitis and cranial neuritis. One wonders how many of these individuals had connective tissue disease. A less common form of idiopathic trigeminal sensory neuropathy with which we have limited experience has an acute onset and a tendency to resolve completely or partially, in much the same manner as Bell’s palsy, with which it is sometimes associated (Blau et al). A recurrent variety of acute trigeminal symptoms of uncertain origin has been reported in the dental literature. We have had experience with two patients whose facial numbness was a component of an upper cervical disc syndrome that included numbness on the same side of the body; presumably the cervical spinal trigeminal nucleus or tract was compressed. Facial numbness, of course, also occurs with diverse conditions such as syringomyelia that affect the spinal nucleus of the trigeminal nerve but there are additional signs of brainstem or upper cervical cord disease.
An idiopathic pure unilateral trigeminal motor neuropathy is known but is a clinical rarity. Chia described five patients in whom an aching pain in the cheek and unilateral weakness of mastication were the main features. Electromyography (EMG) showed denervation changes in the ipsilateral masseter and temporalis muscles. The outcome was favorable.
In most cases of trigeminal neuropathy, except those caused by tumor, herpes zoster, and demyelination, the results of gadolinium-enhanced MRI are normal, as is the cerebrospinal fluid (CSF). The function of the nerve may be studied by the recording of blink reflexes. A few laboratories have developed an evoked potential test specifically of the trigeminal nerve.
The seventh nerve is mainly a motor nerve, supplying all the muscles concerned with facial expression on one side.
The sensory component is small (the nervus intermedius of Wrisberg); it conveys taste sensation from the anterior two-thirds of the tongue and, variably, cutaneous sensation from the anterior wall of the external auditory canal. The taste fibers at first traverse the lingual nerve (a branch of the trigeminal mandibular) and then join the chorda tympani, which conveys taste sensation via the facial nerve to the nucleus of the tractus solitarius. Secretomotor fibers originate in the superior salivatory nucleus and innervate the lacrimal gland through the greater superficial petrosal nerve and the sublingual and submaxillary glands through the chorda tympani (Fig. 47-3).
Figure 47-3.
Scheme of the seventh cranial (facial) nerve. The motor fibers are represented by the heavy blue line. Parasympathetic fibers are represented by regular dashes; special visceral afferent (taste) fibers are represented by long dashes and dots. A, B, and C denote lesions of the facial nerve at the stylomastoid foramen, distal to the geniculate ganglion, and proximal to the geniculate ganglion. Disturbances resulting from lesions at each of these sites are described in the text. (From Carpenter MB, Sutin J: Human Neuroanatomy, 8th ed. Baltimore, Williams & Wilkins, 1982, by permission.)
Several other anatomic facts are worth noting. The motor nucleus of the seventh nerve lies ventral and lateral to the abducens nucleus, and the intrapontine fibers of the facial nerve partly encircle and pass dorsolaterally to the abducens nucleus before emerging from the lower pons, just lateral to the corticospinal tract. The impression made by these looping fibers of the seventh nerve is visible in the floor of the upper fourth ventricle as a protuberance, the facial colliculus. In this region of the pons, infiltrative lesions affect the sixth and seventh nerves simultaneously.
The facial nerve enters the internal auditory meatus with the vestibulocochlear nerve bundle and then bends sharply forward and downward around the anterior boundary of the vestibule of the inner ear. At this angle (genu) lies the sensory ganglion (named geniculate because of its proximity to the genu). The nerve continues in its own bony channel, the facial canal, within which, just distal to the geniculate ganglion, it provides a branch to the pterygopalatine ganglion, i.e., the greater superficial petrosal nerve, which exits the skull through the vidian canal and innervates the lacrimal, nasal, and palatine glands. Somewhat more distally, it gives off a small motor branch to the stapedius muscle and is then joined by the chorda tympani, which projects to the submandibular ganglion and in turn, the submandibular and sublingual glands. The motor root of the facial nerve exits the skull at the stylomastoid foramen and then passes through the parotid gland and subdivides into five branches that supply the facial muscles, the stylomastoid muscle, the platysma, and the posterior belly of the digastric muscle.
A complete interruption of the facial nerve at the stylomastoid foramen paralyzes all muscles of facial expression on the same side. The corner of the mouth droops, the creases and skin folds are effaced, the forehead is unfurrowed, the palpebral fissure is widened and the eyelids will not close completely. Upon attempted closure of the lids, both eyes roll upward (Bell phenomenon), but the one on the paralyzed side remains visible because of lack of eyelid closure. The lower lid sags also, and the punctum falls away from the conjunctiva, permitting tears to spill over the cheek. (In contrast, the paralyzed frontalis muscle in patients of Asian origin sometimes lowers the eyelid and makes the palpebral fissure appear narrowed.) Food and secretions collect between the teeth and cheek, and saliva may dribble from the corner of the mouth. The patient complains of heaviness or numbness and sometimes an aching pain in the face, but sensory loss can usually not be demonstrated. Taste, however, is intact because the chorda tympani has separated from the main trunk of the facial nerve proximal to the stylomastoid foramen.
If the lesion is in the facial canal above the junction with the chorda tympani but below the geniculate ganglion, all the preceding symptoms are present but in addition, taste is lost over the anterior two-thirds of the tongue on the same side. The nerve to the stapedius muscle is also usually involved with a lesion at this site and there is hyperacusis (sensitivity to sudden loud sounds). If the geniculate ganglion or the motor root proximal to it is damaged, lacrimation and salivation may be reduced. Lesions at this point may also affect the adjacent eighth nerve, causing deafness, tinnitus, or dizziness.
The most common disease of the facial nerve is Bell’s palsy (incidence rate of 23 per 100,000 people annually according to Hauser et al). The disorder affects men and women more or less equally and occurs at all ages and all times of the year. There is controversy regarding an increased incidence in women during the third trimester of pregnancy, particularly in the 2 weeks preceding delivery and in the first 2 weeks postpartum; up to a threefold increase has been cited by some authors, but others have failed to find this disproportion. Bell’s palsy is probably more common in diabetic patients and possibly in those with hypertension than in the healthy population.
Regarding the causation of Bell’s palsy, a viral agent has long been suspected, as was discussed by Baringer, any such a mechanism has been established with a reasonable certainty for the majority of cases. Burgess and colleagues identified the DNA of herpes simplex virus (HSV) in the geniculate ganglion of an elderly man who died 6 weeks after the onset of Bell’s palsy. Murakami and coworkers (1996), using the polymerase chain reaction, found HSV type I in the endoneurial fluid surrounding the seventh nerve in 11 of 14 cases of Bell’s palsy; the fluid was obtained during surgical decompression of the nerve in severe cases. The same investigators produced facial paralysis by inoculating HSV into the ears and tongues of mice; virus antigens were then found in the facial nerve and geniculate ganglion. Varicella zoster virus (VZV) was not found in any of their patients but was isolated from patients with the Ramsay Hunt syndrome, which overtly follows shingles (see further on). Patients with fracture or other infections of the temporal bone yielded neither HSV nor VZV gene sequences. In the light of these findings, the term idiopathic facial paralysis, until now the accepted synonym for Bell’s palsy, is not entirely appropriate. As one might expect, the opportunity to examine the facial nerve in the course of Bell’s palsy occurs very rarely. Only a handful of such cases are on record, all showing varying degrees of degeneration of nerve fibers. One case was said to show inflammatory changes, but these may have been misinterpreted (see Karnes).
The onset of Bell’s palsy is acute; about one-half of cases attain maximum paralysis in 48 h and practically all within 3 or 4 days. Pain behind the ear may precede the paralysis by a day or two and in a few patients is intense and persistent. Although a report by the patient of fullness or numbness in the face is common, in a small number there is hypesthesia in one or more branches of the trigeminal nerve. The explanation of this finding is not clear. Impairment of taste is present in most patients but it rarely persists beyond the second week of paralysis. This indicates that the lesion has extended proximal to the point at which the chorda tympani joins the facial nerve. Hyperacusis or distortion of sound is then experienced in the ipsilateral ear and, as mentioned, indicates paralysis of the stapedius muscle.
The facial nerve in Bell’s palsy often displays abnormal signal on gadolinium-enhanced MRI although this may be difficult to appreciate in axial sections if the change is in the vertical part of the facial canal. There is a mild increase of lymphocytes and mononuclear cells in the CSF in a few instances. Cases with more pronounced contrast enhancement of the facial nerve apparently have a worse prognosis (Kress). The enhancement presumably reflects inflammation and swelling along the course of the facial nerve.
Fully 70 percent of patients recover completely within a month or two and 85 percent achieve near-normal facial function, as reviewed by Gilden. Recovery of taste precedes recovery of motor function; if taste returns in the first week, it is a good prognostic sign. But early recovery of some motor function in the first 5 to 7 days is the most favorable sign. EMG may be of value in distinguishing temporary conduction defects from a pathologic interruption of nerve fibers; if there is evidence of denervation after 10 days, one may expect a long delay in the onset of recovery, measured in terms of months. Recovery then proceeds by axonal regeneration, a process that may take 2 years or longer and is often incomplete.
Bell’s palsy recurs in approximately 8 percent of cases in several series (van Amstel and Devriese; Pitts et al), presumably as a result of reactivation of the latent herpes virus. The palsy reemerges during an infection or pregnancy, or for no apparent reason. The interval between episodes is unpredictable but has been 10 years, on average. Recurrent forms of facial paralysis also occur with Lyme disease and sarcoidosis, and in a familial variety as mentioned below.
Protection of the eye during sleep is generally employed in the management of Bells’ palsy. There is no evidence that surgical decompression of the facial nerve is effective, and it may be harmful. The administration of prednisone (40 to 60 mg/d, or an equivalent corticosteroid) during the first week to 10 days after onset has been beneficial in several trials. These medications are thought to decrease the possibility of permanent paralysis from swelling of the nerve in the tight facial canal.
The finding of viral genome surrounding the seventh nerve suggested that antiviral agents might be useful in the management of Bell’s palsy but most evidence from large randomized trials, particularly the one conducted by Sullivan and colleagues, fails to support the use of these drugs alone or in combination with steroids The numerous earlier studies suggesting benefit from the combined antiviral and steroid treatments must be viewed in the context of these larger prospective trials. In appropriate circumstances, testing should be undertaken for infectious causes that would require alternative therapy (e.g., Lyme, HIV, and perhaps mycoplasma) but this is not routinely required. The treatment of facial palsy caused by VZV (Ramsay Hunt syndrome) with antiviral drugs is discussed later.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree