Figure 88.1. Electronic microscopy showing the external membrane of a chronic subdural hematoma. An ample hair light (l) with erythrocytes is visible within its interior (EC), a thin layer of endothelial cells rests on a discontinuous basal membrane (X 5000).
N = nucleus; P = protrusion.
Recent studies have demonstrated that the hematoma content is characterized by elevated levels of cytokines and endothelium-derived growth factors that can contribute to the formation and development of membranes, and considerable amounts of inflammation mediators and fibrinolytic factors that can concur in the genesis of hematoma. Therefore, it seems that the hematoma itself is responsible for its chronic evolution.
Since CSDH develops in the atrophic brains of adults of advanced age, the intracerebral pressure (ICP) usually remains within normal limits or rises slightly. Positron emission tomography (PET) studies that evaluated changes in metabolism and cerebral blood flow (CBF) are contradictory. Ikeda found a local reduction in CBF in hemiparetic patients. Ishikawa demonstrated an increase in cerebral oxygen extraction in the hemisphere adjacent to the hematoma, accompanied by a marked reduction in CBF and cerebral metabolic oxygen rate (CMRO2) mainly in the frontal and temporal lobes and in the basal ganglia.
Recently, Yamada et al. observed a small bilateral reduction in CBF and CMRO2, while oxygen extraction, cerebral blood volume, and cerebrovascular reactivity remained stable. All the altered variables stabilized within 4 weeks after hematoma evacuation. This period is shorter (average 3 days), in patients in which the symptoms reverse.
88.5 Clinical Features
The diagnosis of CSDH requires a high degree of suspicion. Due to its multiple forms of presentation, it is known as “the great imitator”. The commonest onset form is an altered mental state (50-70%). Delirium, confusional states, psychotic symptoms or alterations in behaviour are some of the symptoms that often can lead to the wrong diagnosis of psychiatric diseases or dementia.
In less than half of cases consciousness is impaired, varying from confusion to coma. Near to 60% of patients present with focal neurological deficits characterized by a location contralateral to the hematoma, slow evolution, and fluctuations (appearance and disappearance). Sometimes the symptoms are similar to those of a brain tumour patient with signs of elevated ICP such as migraine, vomiting and hemiparesis. Convulsions are infrequent, except in patients with a history of previous seizures. If they do occur, they are almost always associated with large collections. Other manifestations, although less common, include: gait and position disturbances, ataxia, akinesia, rigidity or extrapyramidal syndromes. In some cases, the hemiparesis may be ipsilateral to the hematoma; this can be explained by displacement and compression of the brainstem against contralateral tentorium.
88.6 Role of Neuroimaging Studies in Diagnosis
The neuroimage of election is CT scan because it is not invasive, it is rapid and low cost; in addition, it also allows the evaluation of other intracranial structures. CSDH has a dynamic nature, therefore requires considering this aspect due to the characteristics of the collection change with the time. During acute phase, the hematoma is hyperdense in relation to cerebral parenchyma due to the presence of fresh blood.
In 40% of acute cases, the density can be mixed with hypodense areas. This finding could be due to the fact that bleeding is so recent that part of the blood is not yet coagulated or because of the presence of CSF originating from the arachnoids.
With time, lysis of the collection takes place, so that the hematoma becomes as dense as the cerebral tissue (isodensity). In the chronic phase, beyond the third week, the collection begins to be reabsorbed, so that the hematoma acquires a hypodense aspect. Nevertheless, remembering the concepts explained in the paragraph on pathophysiology, continuous microhemorrhages can produce sometimes a collection with heterogeneous aspect sometimes with mixed areas with different densities due to precipitation of fresh blood in the declivities (Figure 88.2).
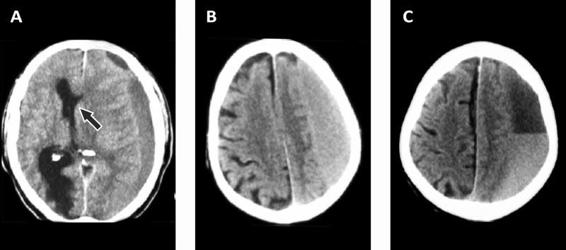
Figure 88.2. (A). Hypodense subdural hematoma with marked midline shift (arrow). (B). Isodense hematoma. (C). Hematoma with mixed density.
Generally, identifying a hematoma is easier when it appears as a hyperdense. The difficulties arise with isodense collections. In such cases, mainly in non trained eyes, it’s very important the analysis of other structures, such as the median line, lateral ventricles and the sulcus between cerebral circumvolutions which often are displaced, compressed or absent, respectively. Another source of confusion is the finding of isodense images after recent trauma. This can be related to very low hemoglobin levels. Bilateral hematomas, usually don’t produce midline shift, but may lead to medial compression of both ventricles, resulting in a narrow, elongated ventricle that can suggest a diagnosis of isodense hematoma (Figure 88.3). In such cases, misdiagnosis may be avoided by injecting contrast material, that reinforce internal membrane of the hematoma, cortical sulcus and vessels.
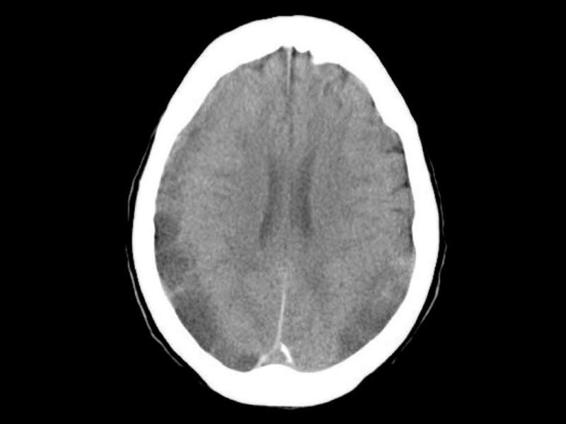
Figure 88.3. Bilateral subdural hematoma with isodense areas.
MRI can be especially useful in patients with isodense hematoma without impairment of other cerebral structures or in those cases with hematoma localized at the vertex, the base of the skull or posterior fossa. In addition, MRI allows the identification of small or transversally oriented collections. CSDH is hyperdense on T1 and T2 sequences, reflecting acute bleeding within a chronic collection. On T1 images it can be isodense (Figure 88.4).
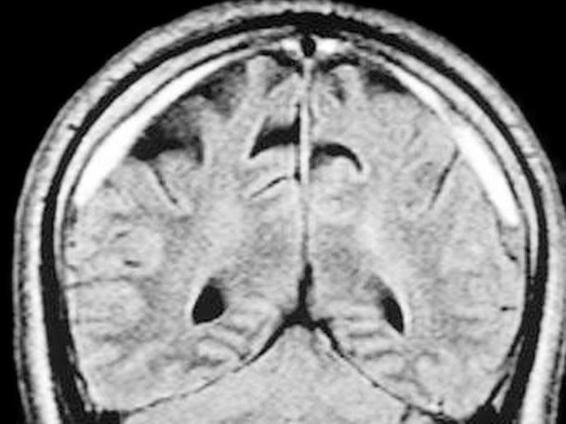
Figure 88.4. T1-weighted image of CSDH.
88.7 Treatment
88.7.1 Surgery
Chronic subdural chronic hematoma is a surgical emergency, although conservative treatment has been successful in some cases. There is nearly unanimous agreement among neurosurgeons that hematoma evacuation is the gold standard mainly in symptomatic cases or when the thickness of the collection is >1 cm (on the tomograph scale); nevertheless, consensus on the surgical technique is lacking. Two recent surveys in Canada and the United Kingdom support this affirmation. Different options exist. Here we will list them, without entering into a detailed description.
- Twist-drill craniotomy. If the drainage orifice is small and drainage is not left, the index of recurrence is increased.
- Burr hole trephination (two or three), for which irrigates normal saline solution and causing the washing of the collection, until the collection is washed out, giving the brain time to re-expand. A closed drainage system can be left, but some still prefer the technique of “last points”.
- Conventional osteoplastic craniotomy, with extirpation of the external membrane of hematoma. Deep membrane is not extirpated, because is attached to the brain and may damage it.
- Neuroendoscopic treatment
- Terphination, making a great hole, through which washing is introduced.
Data from evidence-based medicine do not allow to establish standard treatment guidelines for the surgical treatment of CSDH. Neither trials nor information exist that demonstrate which type of management is better, even if some recommendations are possible; all have a low level of evidence (C):
- Both burr hole trephination and twist-drill craniotomy are safe methods.
- Burr hole trephination has a better treatment/complication ratio and lower recurrence rates than twist-drill craniotomy.
- Conventional osteoplastic craniotomy and burr hole trephination have lower recurrence rates.
- Closed drainage systems reduce the risk of recurrence without increasing the risk of other complications.
88.7.2 Neurointensive Care
Although the treatment of CSDH is generally surgical, almost 20% of cases can resolve spontaneously, principally due to a collections of lower size. If one decides to wait, it is essential to closely monitor the state of consciousness, performing neuroimaging (CT or MR) at the minimum sign of neurological deterioration. Perioperative management doesn’t differ from the management of other neurocritical illnesses, except for some controversial aspects that we will analyze below.
Position of the Head: 0 or 30 Degrees?
In medicine in general, and intensive care in particular, there are dogmas that are handed down from generation to generation, even with the lack of solid scientific evidence. One of these controversial topics is how the head should be maintained after CSDH evacuation. Several opinions among neurosurgeons exist. Some advocate a horizontal (0 degrees) head position, others an elevated position, and still others suggest alternating the positions throughout the day. Advocates of the horizontal position base their rationale on “physical reasons”, because, in theory, this position diminishes the potential space (which can be increased by the cerebral atrophy that accompanying ageing) where hematoma could develop. In addition, horizontal position annuls the effect of the gravity, improves cerebral perfusion and favours cerebral expansion due to vigorous transmission of arterial compression waves within cerebral parenchyma,. Finally, the horizontal position theoretically improves the drainage of subdural collections.
Some neurosurgeons systematically elevate the head to 30 degrees. It is well known, and it has been previously discussed in other chapters of this book, that elevating the head favours the reduction of intracerebral pressure (ICP), facilitating the venous drainage of the brain. Additionally, remember that two theories try to explain the growth of subdural hematoma: the osmotic theory and the theory of recurrent bleeding. Both these components disappear, at least hypothetically, when the head is elevated because the necessary pressure gradient for the development of the osmotic theory diminishes and the action of gravity decreases the intraluminal pressure from the neoformation vessels of the external membrane of the hematoma that originate recurrent bleeding. Other no less important reasons support the choice for head elevation. Gastrointestinal system of the neurocritically ill patients does not work correctly, becoming paralyzed or slowed down due to cerebral damage or side effects of the drugs commonly administered (e.g., morphine, fentanyl, diphenylhydantoin, noradrenaline, etc.). In addition, the use of acid blockers such as omeprazole and lanzoprazole, or blockers of the acid secretion of the stomach like ranitidine, elevate the pH, thus favouring the growth of micro-organisms, mainly Gram-negative species. Also, the lower oesophageal sphincter, whose main function is to prevent gastric reflux, doesn’t work properly. All these aspects can augment the risk of continuous microaspirations, which is one of the main risk factors in the pathogenesis of ventilation-associated pneumonia. This risk remarkably decreases when the head of the bed is raised to 30 degrees or more.
In conclusion, there is no consensus on head position during the postoperative period. Nakajima reported that the rate of recurrence is the same for both positions, whereas Miele et al. found no differences in the rate of expansion or in the recurrence of hematoma.
Corticosteroid Therapy: Yes or No?
Anecdotal series, with a small number of patients, or single case reports suggest a favourable effect of corticosteroid therapy in conservative treatment. The rationale for the use of corticosteroids in CSDH lies in its capacity to decrease the permeability and its anti-angiogenic properties over the subdural clot membrane, both mechanisms derived from experimental studies and very few clinical studies. Data obtained from a retrospective series suggests that dexamethasone (4 mg every 8 hours) is at least a feasible and safe option in the management of CSDH, however, no evidence for their efficacy exists since the studies were not validated in large-scale populations or in randomized controlled trials.
Anticonvulsants
Seizures as presentation form of CSDH are rare with reported rates around 6%. During postsurgical period the data reported are contradictory. The incidence of seizures appears to be inversely related to the population size. In a small series of 50 subjects, the estimated incidence was 32%, whereas the average rate was 3 and 0.3% in two studies involving 568 and 1439 patients, respectively. Since all the reported studies were retrospective, the systematic use of anticonvulsants cannot be recommended. We use anticonvulsants agents in the following circumstances:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






