Chronobiology
Margaret N. Shouse
Mark S. Quigg
Introduction
The term chronobiology refers to the clocking of biologic events. There are many biologic clocks that control behavioral and physiological processes.54 Epilepsy is affected by biologic clocks, a phenomenon that has been documented for more than a century.22,24,33,34,35,57,82,83
This chapter focuses on three kinds of clocks or periodicities as they pertain to epilepsy: (a) circadian rhythms, which recur at about 24-hour intervals, (b) ultradian rhythms, which recur at <24-hour intervals, and (c) infradian rhythms, which recur at >24-hour intervals (e.g., monthly or seasonal).
More than one periodicity can exist. For example, some patients have 24-hour seizure peaks, whereas most have multiple peaks within 24 hours, sometimes at about 90-minute intervals defined by the basic rest–activity cycle (BRAC). Many patients with circadian and ultradian seizures also exhibit strong infradian patterns, as evidenced by multiple cyclic variations in hormonal secretions (Chapters 194,195,196,197,198). The way in which these periodic rhythms affect epilepsy depends on several interrelated variables that define epileptic syndromes,15 including (a) seizure type, (b) etiology, (c) age-dependent course, and (d) prognosis.
Circadian (24-Hour) Rhythms/Variations and Seizure Events
A circadian rhythm in the strict sense refers to any periodicity (hormones, temperature, mobility, etc.) that fluctuates over the course of approximately a 24-hour day and does not necessarily refer to the sleep–wake cycle. The sleep–wake cycle is a circadian rhythm in many mammals, such as rats and humans, but it recurs several times a day in cats. The “time of day” difference does not alter the fact that interictal discharges (IIDs) and clinically evident seizures exhibit similar distributions during different sleep and waking states in all these species. Accordingly, most studies of epilepsy have focused on variables that exhibit variation in the sleep–wake cycle without examining the role of circadian “time of day” rhythms per se.
There are several reasons for the paucity of clear-cut data on circadian rhythms. Perhaps the most relevant is that, in contrast to the sleep–wake cycle, circadian periodicity is not directly measurable because it is not simply a matter of clock time. It requires robust circadian markers or reference points, the use of various mathematical constructs, and data collection over prolonged sampling intervals. True, endogenous circadian rhythms recur on a “free-running” basis regardless of exogenous cues, such as the light–dark cycle, or of any other endogenous factors, such as linkage to sleep versus waking states.
Findings on biologic events that are clearly under circadian rhythm versus sleep–waking state control are difficult to interpret. Masking effects, or perturbation of circadian markers, can complicate interpretation of observations on circadian seizure modulation. For example, cortisol secretion has a circadian periodicity, but seizures themselves elevate cortisol (e.g., Quigg83). Cortisol secretion is also modulated by stress, which is itself a seizure precipitant (e.g., Frucht et al.27). Melatonin is intimately involved in light-related circadian organization of the sleep–wake cycle and, by definition, is susceptible to masking effects by exogenous clues. Exogenous melatonin administration can improve seizure control in experimental animals90 and in clinical trials.23 However, the ameliorative effects of melatonin cannot be dissociated from improvements in sleep, which is disordered in epilepsy (see Chapter 188),44 or from seizure-related factors (e.g., Bazil5).
These issues have been extensively reviewed by Quigg.82,83,84 In this section, we review the evidence on circadian variation in seizure activity as function of the sleep–wake cycle vis-à-vis time of day (clock time) and show examples of endogenous circadian rhythm modulation, when available.
Clinical Findings
Three epilepsy categories are classified according to the timing of generalized myoclonic or tonic–clonic seizures in the sleep–wake cycle. Timing categories are referred to as awakening (diurnal), sleep (nocturnal), and diffuse epilepsies, the latter including epileptic syndromes in which seizures occur randomly in sleep or waking states as well as in the day or night.44,46 These three epilepsy groups are also classified as a function of the different epileptic syndromes with which they are affiliated.15,45,46,75,77
Epilepsies Characterized by Seizures on Awakening (Diurnal): Primary Generalized Epilepsies
Epilepsies characterized by seizures on awakening are a special class of diurnal seizures, which are considered more entrained to the sleep–wake cycle than to endogenous circadian rhythms.31 They are most often primary generalized epilepsies in which the etiology is assumed to be genetic. These include patients with typical absence, juvenile myoclonic epilepsy (JME), and generalized tonic–clonic seizures.2,45,46,47,77
Patients with absence and JME often have generalized tonic–clonic seizures.29,30,48 Greater than 90% of these patients have generalized tonic–clonic convulsions (GTCs) only on arousal from sleep,45,46 most frequently during prolonged drowsy periods occurring between 10 minutes and 2 hours after morning awakening.8,45,46 Myoclonic and absence seizures are also common at this time. Table 1 shows the prevalence of generalized myoclonic seizures after awakening from nocturnal sleep.119
Table 1 Distribution of 51 primary generalized myoclonic seizures in relation to the time of predilection for occurrence of seizures and to the efficacy of treatment in 33 patients | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
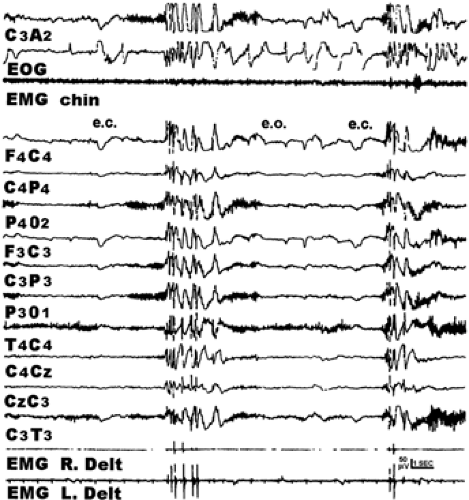
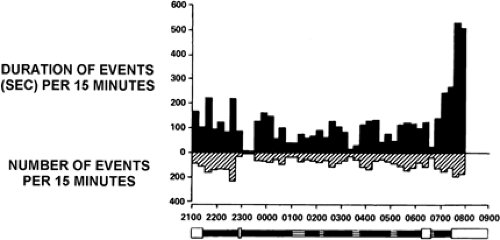
Many patients also exhibit more frequent and prolonged interictal epileptiform discharges after awakening.37,51,52,77,78 Polyspike-and-wave complexes of JME often occur after awakening from nocturnal sleep, as depicted in FIGURE 1.31 FIGURE 2 shows that the duration of 3/sec spike–wave complexes
associated with absence seizure disorders can be longer after awakening than at other times in the sleep–wake cycle.52
associated with absence seizure disorders can be longer after awakening than at other times in the sleep–wake cycle.52
Ictal events are more entrained to awakening than are IIDs. Frequent polyspike-and-wave and spike-and-wave complexes also occur during non–rapid-eye-movement (NREM) sleep (e.g., Fig. 2), even when myoclonic and absence seizures occur only on awakening.
Epilepsies characterized by seizures on awakening typically exhibit an age-dependent clinical course.15,70,73,97,98,99 Onset is usually between 4 and 15 years of age. Spontaneous remission, reduction of seizures, or complete medication control is common between puberty and 20 to 25 years of age, although some patients can be more drug refractory.70,78,98
These relatively benign epileptic syndromes are associated with normal developmental and neurologic functions. The presumed pathophysiology32 is a disturbance in the electrophysiologic and neurochemical response of neocortical cell populations to synchronous synaptic inputs resulting from inhibition of arousal cells located in the hypothalamus and in the ascending reticular activating system (ARAS).4,74,103,104
Epilepsies Characterized by Seizures During Sleep (Nocturnal): Localization-Related Epilepsies
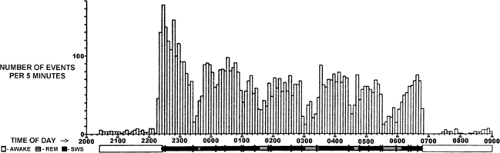
Circadian ictal and interictal discharge patterns occur during the “subjective night” and/or during sleep in epilepsies arising from a focal region of dysfunction (e.g., Frost et al.26). These may be relatively benign or not. For example, some frontal lobe syndromes, notably nocturnal paroxysmal dystonia62,63,94 and autosomal-dominant nocturnal frontal lobe epilepsy (see Chapter 251), display bizarre partial motor seizure manifestations during NREM sleep. The seizures do not secondarily generalize in NREM sleep and are readily controlled by antiepileptic medication. Other “benign” localization epilepsies have an age-dependent course with a specific time frame for onset and spontaneous remission. The prognosis for spontaneous remission of seizure manifestations, is usually good.2,6,7,15,70,78,98,99 These patients also display frequent IID and partial seizures with or without secondary generalization during NREM sleep. Examples are benign partial epilepsy with centrotemporal spikes (BECT, also called benign rolandic epilepsy),58 benign epilepsies with occipital spikes,6,7 Landau-Kleffner syndrome (acquired epileptic aphasia), and patients manifesting electrical status epilepticus during slow sleep (ESES).113 FIGURE 3 shows an example of BECT in which the interictal spike discharge peaks at sleep onset.51
Simple partial or complex partial seizures, particularly those accompanied by secondary generalized tonic–clonic seizures, have long been thought to occur more frequently in sleep than in waking (e.g., Janz45,46). Greater than 50% of patients with temporal or frontal lobe foci reported secondary generalized tonic–clonic seizures only in sleep, whereas complex partial seizures without secondary generalization were reported to occur more often during waking.45,46 Because localization-related epilepsies are more likely to be symptomatic than are primary generalized epilepsies,15,45,46,78 it is not surprising that the prognosis is not always benign. For example, complex
partial seizures of temporal or frontal lobe origin do not have an age-dependent course. Onset frequently occurs before the age of 20 years, spontaneous remission is rare, and seizures are often drug refractory.15,45,46,78
partial seizures of temporal or frontal lobe origin do not have an age-dependent course. Onset frequently occurs before the age of 20 years, spontaneous remission is rare, and seizures are often drug refractory.15,45,46,78
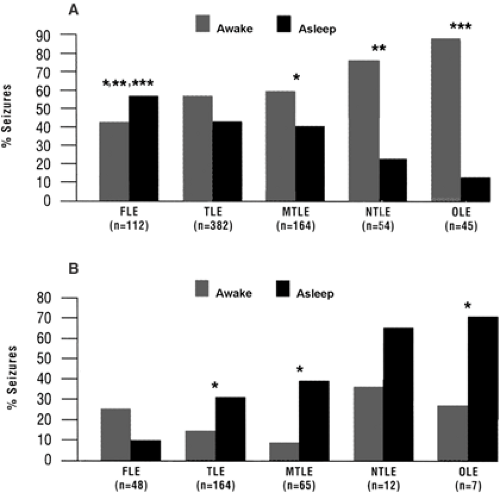
Recent findings using continuous electroencephalogram (EEG)-video monitoring for presurgical evaluation have revealed some interesting new developments in drug-refractory localization-related epilepsies.17,39,48,81 Different timing patterns emerge when patients with drug-refractory complex partial seizures are evaluated as a function of the presence or absence of secondary generalized seizures and as a function of foci in frontal, temporal parietal, or occipital lobes (Fig. 4).40 Partial seizures occur more frequently in waking than in sleep, regardless of the location of the seizure focus (Fig. 4A). Complex partial seizures with secondary generalization occur significantly more often in NREM than in waking at all seizure foci except in frontal lobe (Fig. 4B). It was concluded that different mechanisms govern the timing of seizure initiation versus propagation.
Quigg proposed that circadian diurnal rhythms govern the initiation of symptomatic partial seizures with limbic foci, whereas nocturnal sleep-related mechanisms trigger onset of focal seizures in symptomatic focal seizure disorders with nonlimbic foci. Several findings have been assembled to address this hypothesis:
The timing of symptomatic limbic seizures (mesial temporal lobe sclerosis) can be differentiated from that of nonlimbic (extralimbic) seizures with respect to linkage with circadian versus predominantly vigilance-related seizures. Table 2 summarizes this difference on the basis of recent studies dating from 1998 to 2004.84
Figure 583 illustrates the 24-hour distribution of seizures in two patients with left hippocampal sclerosis and temporal lobe onset of seizures (Fig. 5A, B) and in a single patient with dual pathology (Fig. 5C, D). The three patients with symptomatic mesial temporal lobe epilepsy (MLTE) (Fig. 5A–C) all showed complex partial seizures during the subjective day, whereas the timing of symptomatic, parietal lobe seizures occurred nocturnally and during sleep (Fig. 5D).
Figure 6 contrasts the absence of a circadian rhythm in extratemporal focal seizures (Fig. 6A) with the presence of a circadian seizure distribution in an animal model and in patients with MTLE.83 Findings are plotted as a function of clock time in humans (Fig. 6A, B) and with reference to the circadian temperature cycle in the 12-hour/12-hour light/dark cycle in animals (Fig. 6C). A comparison of findings in Figures 6B and 6C supports the conclusion that MLTE in both species is regulated by a circadian rhythm.82,84,87 This circadian pattern was also maintained in rats during constant darkness when plotted in relation to the free-running circadian temperature cycle (Fig. 6D).87
The interpretation of these findings is complicated by the fact that rats are nocturnally active and humans are diurnally active. Thus, seizure activity seems dissociated from circadian
rest–activity rhythm regulation. In addition, circadian entrainment disappeared when the data from free-running rhythms were plotted with reference to circadian rest–activity patterns (not shown in Fig. 6). Both discrepancies could suggest an underlying circadian modulation of limbic seizure occurrence that does not involve rest–activity or sleep–wakefulness patterns.
rest–activity rhythm regulation. In addition, circadian entrainment disappeared when the data from free-running rhythms were plotted with reference to circadian rest–activity patterns (not shown in Fig. 6). Both discrepancies could suggest an underlying circadian modulation of limbic seizure occurrence that does not involve rest–activity or sleep–wakefulness patterns.
Table 2 Distribution of focal seizures by location of seizure | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Epilepsies in Which Seizures Occur Randomly (Diurnal and Nocturnal): Symptomatic Generalized Epilepsies
In these epileptic syndromes, ictal and interictal discharges occur in all sleep and waking states.45,46,67 This random seizure distribution is often associated with diffuse, severe cerebral dysfunction.43,52,79,97 Three well-known examples are West,14,123 Lennox-Gastaut,30,79 and progressive myoclonus36 syndromes.
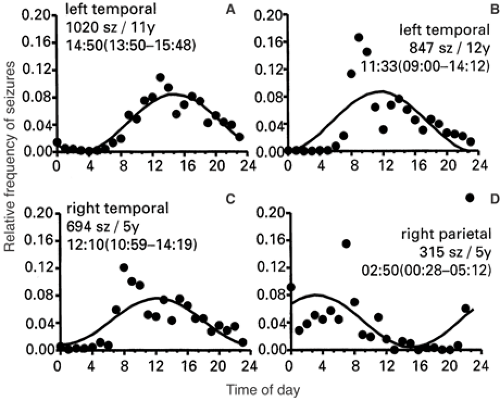 FIGURE 5. Daily distribution of seizures revealed by detailed, long-term diaries maintained by three subjects with medically refractory epilepsy and their spouses. Localization of seizures was later confirmed during epilepsy surgery evaluation. Curves provide the best-fit estimate of time of peak occurrence (95th confidence interval). A, B: Patients with left hippocampal sclerosis and left temporal lobe seizures. C, D: Patient with a pattern of seizures with different symptomatology and a dual pathology: Right temporal lobe seizures associated with ipsilateral hippocampal sclerosis occurred diurnally (C), and parietal lobe seizures associated with a cortical malformation occurred nocturnally and out of phase with temporal seizures (D). (Composite from Quigg83 as follows: Panels A, B from Quigg M. Seizures and circadian rhythms. In: Bazil CW, Malow BA, Sammaritano MR, eds. Sleep and Epilepsy: The Clinical Spectrum. Amsterdam: Elsevier; 2002:127–142; panels C, D adapted from Quigg M, Straume M. Dual epileptic foci in a single patient express distinct temporal patterns dependent on limbic versus nonlimbic brain location. Ann Neurol. 2000;48:117–120; with permission.) |
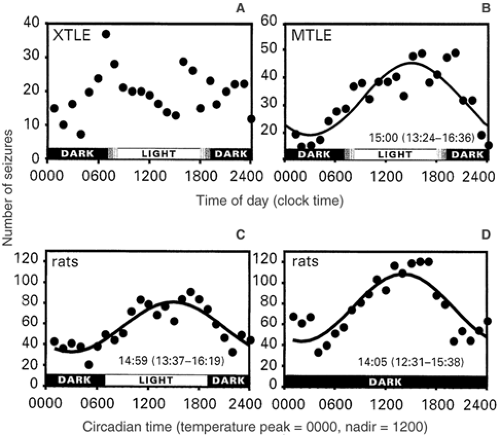 FIGURE 6. Distribution of spontaneous epileptic seizures in humans and in the self-sustained electrical status epilepticus rat model of limbic epilepsy. The times of seizure occurrence in humans were determined using continuous video-electroencephalogram (EEG) recordings from either scalp or intracranial electrodes. In rats, continuous EEG and hippocampal depth electrodes provided times of seizures. Where appropriate, data were fitted using cosinor-nonlinear least squares analysis. A: A biphasic distribution of seizures in patients with extratemporal lobe partial epilepsy (XTLE). When the observed distribution is compared with the expected uniform rate of seizure occurrence using chi-square analysis, the distribution is statistically random. In contrast, limbic seizures in humans with mesial temporal lobe epilepsy (MTLE) (B) and in rats (C, D) occur in similar patterns. Seizures of limbic origin are accurately modeled by a cosine function with the calculated time of peak occurrence reported with the 95% confidence limit. Both species have diurnally predominant seizures despite the fact that rats are nocturnal and humans are diurnal in activity. The limbic seizures of rats occur in similar distributions when entrained to a 12-hour/12-hour light/dark cycle (C) as compared to free-running circadian rhythms in constant darkness or a 12-hour/12-hour dark/dark cycle (D). Note that the times of seizure occurrence are normalized to a 24-hour circadian clock provided by each animal’s circadian rhythm of temperature because, once in constant darkness, the period of circadian rhythms may run slightly shorter or longer than exactly 24 hours. The plot in panel D confirms that the occurrence of limbic seizures is modulated in an endogenous, circadian fashion. (Composite from Quigg M. Seizures and circadian rhythms. In: Bazil CW, Malow BA, Sammaritano MR, eds. Sleep and Epilepsy: The Clinical Spectrum. Amsterdam: Elsevier; 2002:127–142; as adapted from Quigg M, Clayburn H, Straume M, et al. Epilepsia. 2000;41:502–509; and Quigg M, Straume M. Dual epileptic foci in a single patient express distinct temporal patterns dependent on limbic versus nonlimbic brain location. Ann Neurol. 2000;48:117–120; with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|