INTRODUCTION
It is now 30 years since the description of human immunodeficiency virus (HIV) as the cause of acquired immunodeficiency syndrome (AIDS) [1, 2]. The intervening years have witnessed a revolution in antiretroviral (ARV) therapeutics. Infection with HIV no longer heralds inexorable progression to AIDS and death; rather, in resource-rich settings, the infection can be controlled with combined antiretroviral therapy (cART).
HIV infection has become a chronic disease in the post-cART era; it cannot yet be eradicated. Halting cART results in the re-emergence of the virus and, if unchecked, immune compromise. Complications associated with cART-treated-HIV differ from those that manifested previously – opportunistic infections are now rare except in the setting of immune restoration. Whilst the onset of complications is often more insidious than in pre-cART times, they remain associated with significant morbidity – for example, accelerated cardiovascular disease or abnormal bone metabolism [3, 4]. The consequences of decades of living with cART-treated-HIV are not known. Physicians should be cognizant that this remains a dynamic area.
Early in the epidemic, HIV was noted to be both lymphotropic and neurotropic. Not only did neurological disease manifest from central nervous system (CNS) opportunistic infection but also from the direct effects of HIV. Prior to cART, the prevalence of cognitive impairment in HIV-infected patients steadily increased with decline in CD4 T cell count. By the time of death from AIDS, approximately 20–30% of patients were demented [5]. HIV-1-associated dementia (HAD) is phenotypically different from the common dementia of old age – Alzheimer’s disease: it is characterized by an initial subcortical pattern of dysfunction with prominent behavioural and motor symptoms. The first available antiretroviral therapy (ART), zidovudine (ZDV), was associated with improvement in cognitive function, although not with prevention of disease progression when used alone. Subsequent early combinations of ART caused significant side effects through toxicity, the most pertinent to the neurologist being peripheral neuropathy. Ongoing virally induced brain inflammation despite cART, drug-induced toxicity and CNS toxicity and their respective interactions with brain ageing form the core hypothetical explanations for observed cognitive deficits in cART-treated HIV patients.
DEFINITION
HIV-associated neurocognitive disorders (HAND) is an umbrella term encompassing all grades of cognitive dysfunction arising from HIV infection. Its definition arose from a consensus meeting in 2007 [6]. It is subdivided into three syndromes: asymptomatic neurocognitive impairment (ANI); mild neurocognitive disorder (MND) and HAD (Table 3.1). Each has specific diagnostic criteria albeit these are in many respects better suited to research than clinical practice. Each requires the exclusion of delirium, opportunistic infection, significant depressive disease, ongoing substance abuse, as well as other causes for progressive cognitive decline. The definitions pertain to HIV-1 infection, which forms the greatest burden of disease worldwide. For HIV-2, which has the greatest prevalence in west Africa, there is sparse data relating to its neurological complications but it appears more benign from the neurological standpoint [7].
Table 3.1 Revised criteria for diagnosis of HIV-1 associated neurocognitive disorders.a.
| ANI | |
| 1. | Acquired impairment in cognitive functioning, involving at least two ability domains, documented by performance of at least 1.0 SD below the mean for age-education-appropriate norms on standardized neuropsychological tests. The neuropsychological assessment must survey at least the following abilities: verbal/language; attention/working memory; abstraction/executive; memory (learning; recall); speed of information processing; sensory-perceptual, motor skills |
| Cognitive impairment does not interfere with everyday functioning | |
| Cognitive impairment does not meet criteria for delirium or dementia | |
| There is no evidence of another pre-existing cause for the MND | |
| MND | |
| 1. | Acquired impairment in cognitive functioning, involving at least two ability domains, documented by performance of at least 1.0 SD below the mean for age-education-appropriate norms on standardized neuropsychological tests. The neuropsychological assessment must survey at least the following abilities: verbal/language; attention/working memory; abstraction/executive; memory (learning; recall); speed of information processing; sensory-perceptual, motor skills |
| 2. | Cognitive impairment produces at least mild interference in daily functioning (at least one of the following): (i) Self-report of reduced mental acuity, inefficiency in work, homemaking or social functioning. (ii) Observation by knowledgeable others that the individual has undergone at least mild decline in mental acuity with resultant inefficiency in work, homemaking or social functioning |
| 3. | The cognitive impairment does not meet criteria for delirium or dementia |
| 4. | There is no evidence of another pre-existing cause for the MND |
| HAD | |
| 1. | Marked acquired impairment in cognitive functioning, involving at least two ability domains; typically the impairment is in multiple domains, especially in learning of new information, slowed information processing and defective attention/concentration. The cognitive impairment must be ascertained by neuropsychological testing with at least two domains 2 SD or greater than demographically corrected means |
| 2. | The cognitive impairment produces marked interference with day-to-day functioning (work, home life, social activities) |
| 3. | The pattern of cognitive impairment does not meet criteria for delirium (e.g. clouding of consciousness is not a prominent feature); or, if delirium is present, criteria for dementia need to have been met on a prior examination when delirium was not present |
| 4. | There is no evidence of another, pre-existing cause for the dementia (e.g. other CNS infection, CNS neoplasm, cerebrovascular disease, pre-existing neurologic disease or severe substance abuse compatible with CNS disorder) |
Abbreviations: SD, standard deviation; ANI, asymptomatic neurocognitive impairment; MND, mild neurocognitive disorder; HAD, HIV-1 associated dementia.
aRef. [6].
EPIDEMIOLOGY
Following the arrival of cART, the incidence and prevalence of cognitive impairment changed significantly amongst the HIV infected. A marked fall in HAD incidence was noted but prevalence increased [8, 9]. Pre-cART, the mean survival from HAD diagnosis was 6 months. On starting cART, some HAD sufferers showed marked improvement in cognitive function; for others, their decline was halted but they were left severely cognitively impaired with what was later termed burnt out disease.
However, whilst the incidence of HAD declined, physicians noted continued complaints of mild cognitive symptoms amongst cART-treated patients. A number of studies have investigated the prevalence of cognitive impairment in contemporary HIV-infected cohorts. Of these, the CHARTER study (CNS HIV Antiretroviral Therapy Effects Research), from North America, is the largest to report to date [10]. This was a multicentre cross-sectional observational study, and it demonstrated a high prevalence of HAND: 52%. In contrast to the pre-cART era, HAD formed only 2% of the cohort; the majority was classified as ANI (33%). Nadir CD4 count was found to be the strongest predictor of impairment. However, the incidence of comorbidities was high – 30% had contributing conditions. Furthermore, of those receiving cART, 44% had a detectable plasma HIV viral load – an unusually high proportion of individuals on cART had evidence of inadequate treatment either through viral resistance and/or lack of adherence. Others have reported lower levels of HAND (≈20%) in individuals with HIV viral load suppressed beneath current levels of detection or subsequent to controlling for confounding factors [11, 12]. Studies in the pre- and post-cART eras that have included data from control groups report rates of cognitive impairment of 16–19% in the HIV-negative individuals using the neurocognitive definitions for HAND – that is, for ANI values with a minimum of two domains lying at least one standard deviation outside age-education corrected norms [13]. In addition, recently published data shows that the incidence of cognitive impairment in early diagnosed and appropriately managed patients differs little from age-matched controls [14]. Furthermore, whilst the long-term outcome of mild cognitive impairment in the elderly with regard to transformation into Alzheimer’s disease is well studied, fewer data exist describing the relationships between ANI, MND and HAD. Neuropsychological follow-up data from the CHARTER study has, to date, only been reported in abstract form. From baseline to 18–42 months follow-up, worsening cognitive function was observed in 23% of subjects and improvement in 17% [15].
Therefore, the prevalence of HAND is not clear. It is likely to vary between cohorts relating to factors such as availability and adherence to cART, incidence of comorbidities and timing of cART initiation. However, even after allowing for these factors, it is still common and milder than in the pre-cART era.
CLINICAL FEATURES
HAND is predominantly a subcortical process but in milder forms of impairment the areas of deficit may not be quite so distinct [16]. In HAD, aphasia, alexia and agraphia are absent: motor disturbance is prominent [17]. However, when it is severe, these differentiating features are lost and there is global impairment. Pre-cART, HAD symptomatology developed over several weeks to months, whilst MND (then named mild cognitive impairment) developed over a longer period, often months. In cART-treated patients, HAD, as well as MND, unfolds over a much longer time period, unless there is pan-resistance to ARV drugs.
Classically, HAD has three areas of abnormality: cognition, motor function and behaviour. Typical symptoms include decreased concentration, forgetfulness, gait unsteadiness, clumsiness and apathy. Often, on examination primitive reflexes, slowing of fine finger movements and impairment of tandem gait are found. The myelopathy that sometimes accompanies HAD is characterized by spastic paraparesis, usually without a definite sensory level [18, 19]. In cART-treated patients, myelopathy is extremely uncommon. Diminished proprioception and vibration sense are dominant over pinprick or light touch deficits. Changes are largely confined to the legs, and the onset is usually over weeks to months. The clinical features of HAND occurring in cART-treated patients are different: they can fluctuate in a bidirectional pattern over months in some patients. Up to 20% of patients can improve or deteriorate over months, seemingly in the absence of any confounding illnesses or changes in ARV drugs [6, 20]. The degree of change, however, is usually relatively small. The second change that has occurred in the cART era is that HAD can now be either active or inactive, that is, the deficit may be fixed representing past injury [21]. The third change that has come about with the introduction of cART is that HAND is now generally milder [22]. Early symptoms more often relate to impaired concentration and speed of information processing than motor dysfunction [23]. However, even mild disease can have a significant impact upon treatment adherence, quality of life, ease of employment and survival [22, 24, 25]. The fourth change is at this stage less certain. There is some evidence that the ‘phenotype’ of HAND may be different. Some clinical features may be starting to change in view of the differences seen on neuropsychological assessment – there may be more cortical features developing as a result of an interaction with the effects of ageing, Alzheimer’s disease and cerebrovascular disease [13]. The potential relationship to Alzheimer’s disease is particularly interesting and important. HAD patients and clearly HIV patients in general may be at an increased risk of Alzheimer’s disease because of increased life span and adverse metabolic factors, for example, hypercholesterolemia and common mechanisms of toxicity [26]. In 2011, it was estimated that 22% of those with HIV infection in the United Kingdom were aged 50 years or more, almost double the proportion one decade earlier [27]. Similar changes in the demographics of HIV-infected cohorts are seen across the resource-rich world – in Australia, the number of men over 60 with HIV is estimated to increase by ≈400% by 2030 [28]. The impact of cART-treated-HIV on the ageing brain is an area of active research.
ASSESSMENT
The management of the HAND patient is evolving. The following is a suggested approach (Figure 3.1). The initial step is to determine whether the disorder is active or inactive. The principal tool by which this is assessed is the history from the patient, the loved one and the family. History taking should be tailored to elicit symptoms described in the previous section. At this stage, the role of depression should be assessed – it can be associated with HAND, or an untreated mimicker of the syndrome. It remains important to exclude opportunistic infection as a cause for cognitive decline in the HIV infected, which is usually possible following brain imaging and cerebrospinal fluid (CSF) examination. Furthermore, the possibility of drug toxicity should be considered. A detailed pharmacological history should be documented. Usually, drug toxicity is problematic shortly after starting a new ARV drug but recent data has demonstrated an association with the non-nucleoside analogue efavirenz in patients stable on treatment for a median of 87 months [29]. In some cases, measurement of ARV drug blood levels may help confirm drug toxicity. A common confound in HAND or coexistent finding is substance abuse. This should be sought from the patient’s history identifying substances and patterns of use. It is important to ascertain objective evidence of cognitive impairment, ideally through comprehensive neurocognitive assessment.
The next step in management hinges on the nature of the activity: it should be possible to assess whether it is progressive, regressive or stable. However, at present better tools are needed to distinguish between inactive HAND and stable HAND – the latter seems to be characterized by evidence of CSF viral and immunological activity that presumably are just keeping each other in check, whilst the former has no evidence of viral or immunological activity in the CSF. Active progressive and stable HAND should have their ARV regimen altered if possible.
When cognitive decline continues despite suppression of virus in both blood and CSF, it is important to reconsider the possibility of a non-HIV-related degenerative process, although there is evidence that HAND may progress despite viral suppression in both compartments, blood and CSF.
INVESTIGATIONS
Routine Laboratory Studies
HIV patients with cognitive impairment should be screened for common metabolic causes of cognitive impairment and delirium including full blood count; vitamin B12 and red cell folate levels; renal, liver, bone and thyroid profiles; blood glucose and C-reactive protein. Syphilis and hepatitis C serologies should be checked as well as routine HIV treatment parameters: T-lymphocyte blood counts and HIV-1 blood viral load.
Imaging Modalities
Either computed tomographic (CT) scanning or magnetic resonance imaging (MRI) of the brain should be performed to exclude a mass lesion such as toxoplasmosis or lymphoma, as well as to address the likelihood of disorders such as cytomegalovirus (CMV) encephalitis and progressive multifocal leukoencephalopathy (PML). MRI is generally superior to CT, especially for the delineation of the latter two entities.
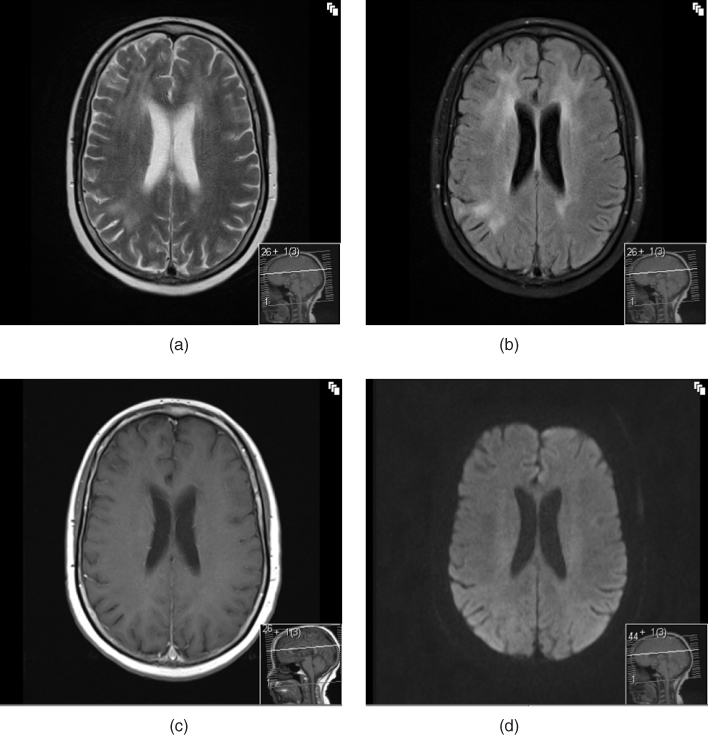
The supportive imaging findings for a HAD diagnosis are as follows: CT scanning of the brain scan usually demonstrates general cerebral atrophy especially of the caudate nuclei [30]. MRI additionally often has T2-weighted patchy or diffuse periventricular abnormalities, usually without corresponding hypointensities on T1 sequences (in contrast to the pattern seen in PML) [31]. These changes are not indicative of demyelination but represent increased interstitial water; they are reversible with cART (Figure 3.2) [32]. However, these findings were defined in patients who were either naïve to ART or who were studied in the pre-cART era: these structural abnormalities are less frequently seen in patients on cART developing HAND. Patchy white matter changes may be seen in ANI or MND but such changes are also seen in HIV negative individuals; hence, their specificity for HAND is low.
Figure 3.2 MRI in HAND: changes of HIV encephalitis. (a) T2 axial image showing white matter change; (b) T2 FLAIR axial image showing white matter change; (c) T1 axial image with gadolinium enhancement showing no corresponding abnormality; (d) diffusion-weighted axial image showing normal appearances.
Magnetic resonance spectroscopy (MRS) is helpful in HAD. There are reduced levels of N-acetyl aspartate, a marker of neuronal function, in the deep frontal white matter and basal ganglia along with elevated levels of myoinositol (found only in glial cells) and choline, a marker that is present in higher concentrations in glial and inflammatory cells reflecting changes in cell membrane injury, turnover or glial cell activation [33]. Following initiation of cART, these changes can normalize although they may lag behind the rise in CD4 cell count and fall in CSF HIV viral load. MRS abnormalities are also reported in milder forms of HAND, in patients with possible ARV neurotoxicity and in cART-naive patients starting ARVs [34–36].
Other MRI techniques such as magnetization transfer ratio and diffusion tensor imaging are beginning to be explored and hold promise in the identification and quantification of fixed damage [37]. Single photon emission computed tomography (SPECT) often shows multifocal cortical and subcortical areas of hypoperfusion, although the changes are very nonspecific [38]. Positron emission tomography, on the other hand, has been found to show areas of hypometabolism in the basal ganglia that are relatively specific for HAD [39]. These imaging modalities are not recommended as part of routine diagnostic work-up for HAND patients.
Cerebrospinal Fluid Studies
CSF analysis may reveal ‘background’ abnormalities that can occur in asymptomatic HIV-infected individuals: a mild mononuclear pleocytosis, a mildly raised CSF protein, intrathecal IgG synthesis and oligoclonal bands [40]. Again, the influence of cART on these abnormalities has not been fully addressed but, in practice, the white cell count and protein levels usually return to the normal range.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree