Chapter 6 Contextual influences
nutrition, respiration and other factors
This text has as its primary focus the manual, biomechanical, evaluation and treatment approaches appropriate to care of dysfunction and pain problems. It is unwise, however, to restrict attention to a simplistic formula that suggests that there are only ‘mechanical solutions for mechanical problems’. A subtext, elaborated on in Volume 1, Chapters 4 and 7 and in this text’s ‘Essential information’ chapter, enunciates the view of complex, rather than simplistic, etiologies for most forms of dysfunction and pain. It is important that contextual influences always be considered, including chronobiological factors, nutrition, endocrine responses, anxiety and breathing patterns.
Implicit in this focus on biochemistry is a need for awareness of the influences of such factors as sleep and breathing patterns on the chemical processes involved in most conditions involving inflammation, pain and the healing of tissues (Adams 1977, Affleck 1996). Also of great importance is the need for the practitioner to operate within the scope of her license and training. Even if the license allows for the practice of counseling these factors, the need for appropriate training and maintenance of current continuing education requirements is important for the provision of optimal clinical care.
Chronobiology
Where inflammation is part of the cause of a painful condition, anything that reduces or modifies the inflammatory process is likely to reduce the level of perceived pain. However, while inflammation may not be pleasant, it is a vitally important process in repairing (or defending against) damage, irritation or infection. Therefore, strategies that try to modify inflammation need to aim at a limited degree of modulation, rather than total elimination of this healing process. See Volume 1, Chapter 7 for further discussion of a variety of influences on local and systemic inflammation.
Before assessing nutritional influences on inflammation and pain, note should be taken of research that demonstrates the existence of diurnal patterns that profoundly influence inflammatory processes and that explains why inflammation, of all sorts, is normally more intense at night. The normal pattern results in inflammatory processes alternating with those aspects of immune functions concerned with defense against infection; however, these diurnal patterns can be disrupted by a number of factors (Petrovsky & Harrison 1998, Petrovsky et al 1998).
Defensive and repair processes, of which inflammation is a part, are more active between roughly 10pm and the following 10am. For example, Sutherland (2005) reports that as many as 75% of asthmatic subjects are awakened by asthma symptoms, at least once per week, with approximately 40% experiencing nocturnal symptoms on a nightly basis. A great deal of research demonstrates that nocturnal symptoms of cough and dyspnea accompany circadian (i.e. chrononbiological) variations in airway inflammation and physiologic variables.
Monro (2001) reports that: ‘A natural cycling between the defensive and repair modes of aspects of the immune system is disturbed in ill-health and a chronic cytokine shift may lock the body into a pro-inflammatory state’.
• exposure to carbamate and organophosphate insecticides, which inhibit interleukin-2, essential for Th1 function
• intake of steroids, such as cortisone
• ‘Stress, both psychological and physical. Stress activates the hypothalamo–pituitary–adrenal axis and leads to increased production of cortisol. Excessive exercise and deprivation of food or sleep also result in a falling ratio of DHEA to cortisol and an increase in a Th1 to Th2 shift. It is known that Epstein-Barr virus antibody titers rise amongst students facing examinations and that this virus is usually controlled by a Th1 response. Stress causes increased viral replication and hence antibody production’ (Monro 2001).
• Cancer. ‘Many of the risk factors for cancer, such as carcinogenic chemicals or tobacco smoke also cause long-term inflammation and lower Th1 levels’ (Monro 2001).
Sleep and pain
Additional to these influences, disturbed sleep patterns can produce negative effects on pain and recovery from injury. Any disruption of stage 4 sleep results in reduction in growth hormone production by the pituitary gland, leading to poor repair of irritated, inflamed and damaged tissues and longer recovery times (Griep 1994, Moldofsky & Dickstein 1999).
‘The interaction of the circadian sleeping-waking brain and the cytokine-immune-endocrine system is integral to preserving homeostasis. … there may be host defense implications for altered immune and endocrine functions in sleep-deprived humans. Activation of cytokines and sleepiness occur during the acute phase response to bacterial or viral disease. There are disturbances in sleep and cytokine-immune functions in chronic protozoal and viral disease… Sleep-related physiological disturbances may play a role in autoimmune diseases, primary sleep disorders and major mental illnesses.’ (Monro 2001)
Pain and inflammation: allergic, dietary and nutritional factors
Inflammation is a natural and mostly useful physical response to irritation, injury and infection. To drastically alter or reduce it may be counterproductive and, therefore, a mistake, as has been shown in the treatment of arthritis using non-steroidal antiinflammatory drugs (NSAIDs) over the past 30 years or so. Apart from the toxic nature of NSAIDs, untreated joints have commonly been shown to remain in better condition than those treated with NSAIDs (Pizzorno 1996, Werbach 1996).
Nutritional approaches for modulating inflammation (Bakker et al 2010, Sanders 2007)
The reasoning behind the importance of antiinflammatory dietary protocols for patients is given below. The advice for the patient (guidelines that can be copied for the patient’s use) is found in Chapter 7 and in the Appendix.
1. Reduce consumption of animal fats. Pain/inflammation processes involve particular prostaglandins and leukotrienes, which are (to a great extent) dependent upon the presence of arachidonic acid, which humans manufacture mainly from animal fats. Reducing animal fat intake cuts down access to the enzymes that help to produce arachidonic acid and, therefore, lowers the levels of the inflammatory substances released in tissues that contribute so greatly to pain (Donowitz 1985, Ford-Hutchinson 1985).
2. Eating fish or taking fish oil helps ease inflammation (Moncada 1986). Fish deriving from cold water areas such as the North Sea or Alaskan waters contain the highest levels of eicosapentanoic acid (EPA), which reduces levels of arachidonic acid in tissues and therefore helps to produce fewer inflammatory precursors. Fish oil provides these antiinflammatory effects without interfering with those prostaglandins, which protect the stomach lining and maintain the correct level of blood clotting. Over-the-counter drugs, such as NSAIDs, which reduce inflammation, commonly cause new problems by interfering with prostaglandin function as well as encouraging gut dysfunction, which may lead to intolerance or allergic reactions (see below).
Research has shown that the use of EPA in rheumatic and arthritic conditions offers relief from swelling, stiffness and pain, although benefits do not usually become evident until supplementation has been taken for 3 months, reaching their most effective level after about 6 months (Werbach 1991a).
Patients (unless intolerant to fish) should be advised to:
3. Antiinflammatory (proteolytic) enzymes, derived from plants, have a gentle but substantial antiinflammatory influence. These include bromelaine, which comes from the pineapple stem (not the fruit), and papain from the papaya plant. Around 2–3 g of one or other should be taken (bromelaine seems to be more effective) spread through the day, away from meal times as part of an antiinflammatory, pain-relieving strategy (Cichoke 1981, Taussig 1988).
Intolerances, allergies and musculoskeletal dysfunction
Specific individualized pathophysiological responses to particular foods and liquids account for a significant amount of symptom production, including pain and discomfort (Brostoff 1992). In order to make sense of a patient’s presenting symptoms, remain alert to the possibility that at least some of the pain, stiffness, fatigue, etc. may be deriving from, or being aggravated by, what is being consumed.
Two different responses seem to be involved: true food allergy, which is an immunological event (involving immunoglobulin E or IgE), and the less well-understood phenomenon of food intolerance, which involves adverse physiological reactions of unknown origin, without immune system intervention. It is possible that food intolerance may include an element of actual food toxicity or a very individual reaction to foods, probably related to enzyme deficiency (Anderson 1997).
Mitchell (1988) states:
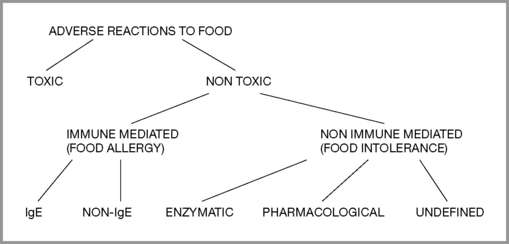
By definition (Royal College of Physicians 1984), food intolerance is a reproducible, unpleasant (i.e. adverse) reaction to a specific food or food ingredient that is not psychologically based (food aversion). Classification of adverse reactions (Ortolani & Vighi 1995) is divided into toxic and non-toxic, with toxic implying general human toxicity, not individual susceptibility. Non-toxic divisions include immune mediated (food allergy) and non-immune mediated (food intolerance). Provocation and other tests help to determine the level of reaction and whether desensitization might be attempted or if total avoidance should be recommended. (Figure 6.1)
Mechanisms
Unfortunately, in many instances food antigens and immune complexes also find their way across this mucosal barrier. How fast and in what quantity such undesirable substances enter the bloodstream from the gut seem to be directly linked to the quantity of antigenic material in the gut lumen (Mitchell 1988, Walker 1981).
• Drugs (antibiotics, steroids, alcohol, NSAIDs – see discussion earlier in this chapter) (Bjarnason 1984, Jenkins 1991)
• Advancing age (Hollander 1985)
• Specific genetically acquired intolerances (allergies)
• Infections and overgrowths in the intestine, e.g. bacterial, yeast (Isolauri 1989)
• Chemicals contaminating ingested food (pesticides, additives, etc.) (O’Dwyer 1988)
• Maldigestion, constipation (leading to gut fermentation, dysbiosis, etc.) (Iacano 1995)
• Emotional stress that alters the gut pH, negatively influencing normal flora
• Major trauma, such as burns (possibly due to loss of blood supply to traumatized area) (Deitch 1990)
• Toxins that are not excreted or deactivated may end up in the body’s fat stores (O’Dwyer 1988)
Research suggests that the relative health and efficiency of the individual’s liver, along with the age of first exposure, the degree of antigenic load and the form in which the antigen is presented, all play roles in deciding how the body responds, with some degree of adaptive tolerance being a common outcome (Mitchell 1988, Roland 1993). Early feeding patterns are one key factor in determining the way the body later responds to antibodies delivered via food and which foods are most involved, with eggs, milk, fish and nuts being among those most likely to produce problems (Brostoff 1992, Mitchell 1988).
Most people exhibit some degree of serum antibody responses to food antigens. Antibodies assist in elimination of food antigens by forming immune complexes, which are subsequently eliminated by the immune system. However, failure to remove such complexes may result in them being deposited in tissues, leading to subsequent inflammation (Brostoff 1992). Sometimes the immune response to food antigens involves IgE and sometimes it does not, in which case the response would attract a label of ‘food intolerance’.
Mast cells, immune responses and inflammation
The violence of any reaction between mast cells and IgE (or other stimuli) depends on the presence in the tissues of a variety of biological substances, such as histamine and arachidonic acid (and its derivatives such as leukotrienes), all of which augment inflammatory processes (Holgate 1983, Wardlaw 1986). Histamine is secreted by mast cells during exposure to allergens and the result is local inflammation and edema as well as bronchiole constriction. This last effect is especially relevant to asthmatics but can affect anyone to some degree, creating breathing difficulties.
At times the response to ingested and absorbed antigens is very fast – a matter of seconds, however, it is also possible for hours or days to elapse before a reaction occurs (Mitchell 1988).
Muscle pain and allergy/intolerance
Copious evidence exists linking allergy and food intolerance to muscle pain, chronic fatigue, fibromyalgia syndrome and a host of other mysterious and complex symptoms. Lactose intolerance produced temporal pain, blurred vision, dizziness and tachycardia in one patient that lasted 26 years before diagnosis. (Matthews & Campbell 2000). Many people suffering perplexing symptoms are often labeled as psychosomatic patients, with the symptoms described as ‘somatoform’ – i.e. symptoms that cannot be traced to a specific physical cause. Rief et al (2001) report that the most frequent somatoform symptoms were back pain, joint pain, pain in the extremities, and headache, as well as abdominal symptoms (bloating or intolerance of several foods) and cardiovascular symptoms (palpitation). The question as to the validity of ‘intolerance’ as a non-psychosomatic condition continues to rage in medical circles. (Nettleton et al 2009) Answers other than ‘somatoform’ or ‘psychosomatic’ are emerging. For example, Fruhauf (2009) notes that:
The manifestation and severity of allergic rhinitis symptoms exhibit marked 24 hour variations; in most people symptoms are worse overnight, or early in the morning, and often compromise nighttime sleep, resulting in poor daytime quality of life, disturbed school and work performance, irritability and moodiness (Smolensky et al 2007).
A study evaluated the frequency of major symptoms as well as allergic symptoms, such as rhinitis, in a group of more than 30 patients with a diagnosis of ‘primary fibromyalgia’ compared with matched (age and sex) controls (Tuncer 1997). Symptom prevalence in the FMS group (apart from pain, which was 100%) was migraine 41%, irritable bowel syndrome (IBS) 13%, sleep disturbance 72% and morning stiffness 69%. There was a frequent finding of allergy history in the FMS group, with elevated (though not significantly) IgE levels. Sixty-six percent of the FMS patients tested were positive for allergic skin tests.
A study at the school of medicine of East Carolina University in 1992, involving approximately 50 people with hay fever or perennial allergic rhinitis, found that approximately half those tested fitted the American College of Rheumatology criteria for fibromyalgia (Cleveland et al 1992).
Four patients diagnosed with fibromyalgia syndrome for between 2 and 17 years, who had all undergone a variety of treatments with little benefit, all had complete, or nearly complete, resolution of their symptoms within months after eliminating monosodium glutamate (MSG), or MSG plus aspartame, from their diet. All patients were women with multiple co-morbidities prior to elimination of MSG. All have had recurrence of symptoms whenever MSG is ingested. The researchers note that excitotoxins are molecules, such as MSG and aspartame, that act as excitatory neurotransmitters and can lead to neurotoxicity when used in excess. They proposed that these four patients may represent a subset of fibromyalgia syndrome that is induced or exacerbated by excitotoxins or, alternatively, may comprise an excitotoxin syndrome that is similar to fibromyalgia (Werbach 1993).
Simons et al (1999) note that patients with active symptoms of allergic rhinitis as well as myofascial trigger points receive only temporary relief when specific therapy is given for the trigger points. ‘When the allergic symptoms are controlled, the muscle response to local TrP therapy usually improves significantly. Hyper-sensitivity to allergens, with histamine release, seems to act as a perpetuating factor for myofascial trigger points.’ They note that food allergies should be considered as a perpetuating factor for myofascial TrPs and that although the ‘shock organs for allergic reactions’ in most people are the upper respiratory tract, eyes, bronchi, skin or joints, ‘in other patients, the skeletal muscles appear to serve as the shock organ for allergies’.
Dr Anne Macintyre, medical adviser to ME Action, an active UK support group for patients with myalgic encephalomyelitis, fibromyalgia and chronic fatigue conditions, supports an ‘immune dysfunction’ model as the underlying mechanism for FMS. She states: ‘The immune dysfunction in ME may be associated with increased sensitivities to chemicals and/or foods, which can cause further symptoms such as joint pain, asthma, headache and IBS’ (Macintyre 1993).
For many years, Dr Theron Randolph recorded clinical changes as an individual passes through stages of ‘reaction’ to chemicals (in food or in the environment) (Randolph 1976). He divides these reactions into those that relate to the active stimulation of an immune reaction by the allergen and those that relate to withdrawal from it. During some of the stages, most notably ‘systemic allergic manifestations’, most of the major symptoms associated with FMS may become apparent, including widespread pain, fatigue, mental confusion, insomnia and irritable bowel. Where particular food allergens are consumed daily, reactions are usually not acute but may be seen to be chronically present. The clinical ecology model suggests that the individual may by then have become ‘addicted’ to the substance and that the allergy is then ‘masked’ by virtue of regular and frequent exposure to it, preventing the withdrawal symptoms that would appear if exposure was stopped. Feingold (1973) states:
Allergy-hyperventilation ‘masqueraders’
Blood chemistry can be dramatically modified (increased alkalosis) by a tendency to hyperventilation and this has profound effects on pain perception and numerous other symptoms including anxiety, sympathetic arousal, paresthesia and sustained muscular tonus (Lum 1981, Macefield & Burke 1991, Timmons & Ley 1994).
Brostoff (1992) states that some experts are actually dismissive of the concept of food intolerance and believe that many individuals so diagnosed are actually hyperventilators. He considers that: ‘Hyperventilation is relatively uncommon and can masquerade as food sensitivity’. Barelli (1994) has shown that a tendency to hyperventilation increases circulating histamines, making allergic reactions more violent and more likely.
Defining food intolerances
In the 1920s and 1930s, Dr A.H. Rowe demonstrated that widespread chronic muscular pains, often associated with fatigue, nausea, gastrointestinal symptoms, weakness, headaches, drowsiness, mental confusion and slowness of thought, as well as irritability, despondency and widespread bodily aching, commonly had an allergic etiology. He called the condition ‘allergic toxemia’ (Rowe 1930, 1972).
Randolph (1976) has described what he terms ‘systemic allergic reaction’, which is characterized by a great deal of pain, either muscular and/or joint related, as well as numerous symptoms common in FMS. Randolph says:
Randolph points out that when a food allergen is withdrawn from the diet it may take days for the ‘withdrawal’ symptoms to manifest: ‘During the course of comprehensive environmental control [fasting or multiple avoidance] as applied in clinical ecology, myalgia and arthralgia are especially common withdrawal effects, their incidence being exceeded only by fatigue, weakness, hunger and headache’. The myalgic symptoms may not appear until the second or third day of avoidance of a food to which the individual is intolerant, with symptoms starting to recede after the fourth day. He warns that in testing for (stimulatory) reactions to food allergens (as opposed to the effects of withdrawal), the onset of myalgia and related symptoms may not take place for between 6 and 12 hours after ingestion (of an allergen-containing food), which can confuse matters as other foods eaten closer to the time of the symptom exacerbation may then appear to be at fault. Other signs that can suggest that muscle pain is allied to food intolerance include the presence of restless legs, a condition that also commonly co-exists with FMS and contributes to insomnia (Ekbom 1960).
If symptoms such as muscular pain may at times be seen to be triggered by food intolerance or allergy, the major question remains – what is the cause of the allergy? (Box 6.1) As discussed earlier in this chapter, one possibility is that the gut mucosa may have become excessively permeable, so allowing molecules to enter the bloodstream where a defensive immune response is both predictable and appropriate. ‘Leaky gut’ can be seen to be a cause of some people’s allergy (Paganelli 1991, Troncone 1994). The trail does not stop there, however, because it is necessary to ask: what caused the leaky gut?
Box 6.1 Biological synchronicity
A different way of viewing two events is to see them as being part of a complex continuum, each being part of the same (larger) process but with neither event dependent on the other, linked by a synchronistic connective principle. The words ‘synchronicity’ or ‘simultaneity’ are used to describe this way of viewing patterns and events (Jung 1973).
• hyperventilation commonly leads to anxiety; therefore, we might assume that hyperventilation ‘causes’ anxiety; however
• anxiety commonly leads to hyperventilation; therefore, we might assume that anxiety causes hyperventilation; or it might be said that
• anxiety and hyperventilation not only ‘feed’ each other but can be triggered and/or aggravated by low blood sugar levels, increased progesterone levels, sympathetic arousal, toxic factors, adrenal stimulation, metabolic acidosis, climatic conditions, altitude, emotional stimuli, allergic reactions and so forth. Therefore, we might more comprehensively and appropriately assume that anxiety and hyperventilation are part of a continuum, involving all or any of these (and numerous other) factors, interacting with the unique genetic and acquired biochemical, biomechanical and psychological individuality of the person affected.
This way of viewing the patient’s problem involves placing it in context: the problem within the patient (in all his/her acquired and inherited uniqueness and complexity), within the patient’s environment, and that environment within the broader environment, etc. This approach can be termed ‘biological synchronicity’ (Chaitow 2001) for if we are looking for ‘causes’ of symptoms we need to think as broadly as possible so that with a wide enough lens, we may discern a pattern, a web of influences, which we may be able to help the patient untangle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree