SECTION V FUNCTIONAL SYSTEMS
CHAPTER 13
Control of Movement
CONTROL OF MOVEMENT
Evolution of Movement
Movement (motion) is a fundamental and essential property of animal life. In simple, unicellular animals, motion depends on the contractility of protoplasm and the action of accessory organs: cilia, flagella, and so forth. Rudimentary multicellular animals possess primitive neuromuscular mechanisms; in more advanced forms of animal life, reflexive motion is based on the transmission of impulses from a receptor through an afferent neuron and ganglion cell to motor neurons and muscles. This same arrangement is found in the reflex arc of higher animals, including humans, in whom the spinal cord has further developed into a central regulating mechanism. Superimposed on these reflex circuits, the brain is concerned with the initiation and control of movement and the integration of complex motions.
Control of Movement in Humans
The motor system in humans controls a complex neuromuscular network. Commands must be sent to many muscles, and multiple ipsilateral and contralateral joints must also be stabilized. The motor system includes cortical and subcortical areas of gray matter; the corticobulbar, corticospinal, corticopontine, rubrospinal, reticulospinal, vestibulospinal, and tectospinal descending tracts; gray matter of the spinal cord; efferent nerves; and the cerebellum and basal ganglia (Figs 13–1 and 13–2). Feedback from sensory systems and cerebellar afferents further influences the motor system.
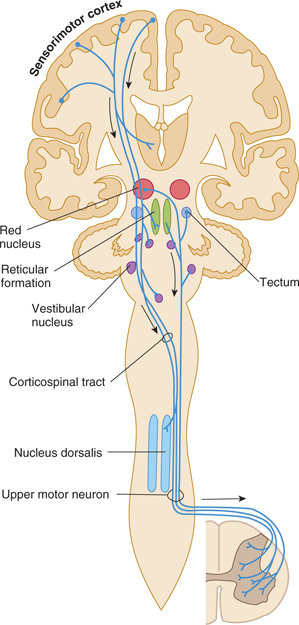
FIGURE 13–1 Schematic illustration of some pathways controlling motor functions. Arrows denote descending pathways.
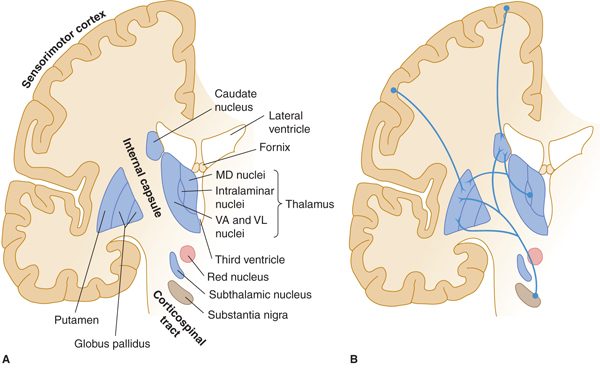
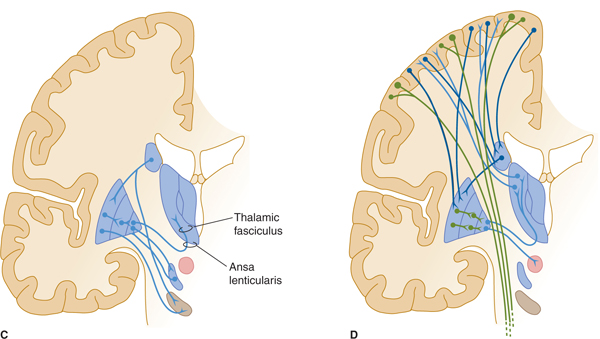
FIGURE 13–2 A: Basal ganglia: major structures. MD, medial dorsal; VA, ventral anterior; VL, ventral lateral nuclei of thalamus. B: Major afferents to basal ganglia. C: Intrinsic connections. D: Efferent connections.
Movement is organized in increasingly complex and hierarchical levels.
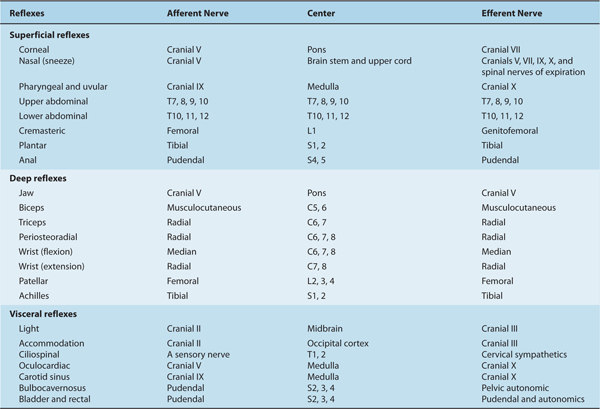
Reflexes are controlled at the spinal or higher levels (Table 13–1; see also Chapter 5).
TABLE 13–1 Summary of Reflexes.
Stereotypic repetitious movements, such as walking or swimming, are governed by neural networks that include the spinal cord, brain stem, and cerebellum. Walking movements can be elicited in experimental animals after transection of the upper brain stem, probably as a result of the presence of central pattern generators, or local circuits of neurons that can trigger simple repetitive motor activities, in the lower brain stem or spinal cord.
Specific, goal-directed movements are initiated at the level of the cerebral cortex.
MAJOR MOTOR SYSTEMS
Corticospinal and Corticobulbar Tracts
A. Origin and Composition
The fibers of the corticospinal and corticobulbar tracts arise from the sensorimotor cortex around the central sulcus (see Fig 13–1); about 55% originate in the frontal lobe (areas 4 and 6), and about 35% arise from areas 3, 1, and 2 in the postcentral gyrus of the parietal lobe (see Fig 10–11). About 10% of the fibers originate in other frontal or parietal areas. The axons arising from the large pyramidal cells in layer V (Betz’s cells) of area 4 contribute only about 5% of the fibers of the corticospinal tract and its pyramidal portion.
The portion of the pyramidal tract that arises from the frontal lobe is concerned with motor function; the portion from the parietal lobe deals more with modulation of the ascending systems. The tracts have endings or collaterals that synapse in the thalamus (ventral nuclei), the brain stem (pontine nuclei, reticular formation, and nuclei of cranial nerves), and the spinal cord (anterior horn motor neurons and interneurons; Fig 13–3). A direct pathway to spinal cord motor neurons exists only for the musculature of the distal extremity, such as the fingers that require rapid and precise control.
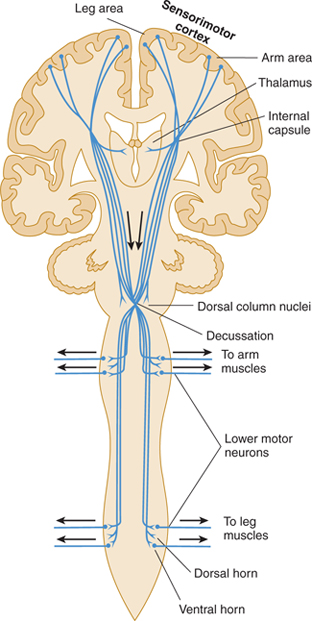
FIGURE 13–3 Diagram of the corticospinal tract, including descending fibers that provide sensory modulation to thalamus, dorsal column nuclei, and dorsal horn.
B. Pathways
The corticobulbar (corticonuclear) fibers originate in the region of the sensorimotor cortex, where the face is represented (see Figs 10–13 and 10–14). They pass through the posterior limb of the internal capsule and the middle portion of the crus cerebri to their targets, the somatic and brachial efferent nuclei in the brain stem. The corticospinal tract originates in the remainder of the sensorimotor cortex and other cortical areas. It follows a similar trajectory through the brain stem and then passes through the pyramids of the medulla (hence, the name pyramidal tract), decussates, and descends in the lateral column of the spinal cord (see Figs 5–13, 13–1, and 13–3). About 10% of the pyramidal tract does not cross in the pyramidal decussation but descends in the anterior column of the spinal cord; these fibers decussate at lower cord levels, close to their destination. In addition, up to 3% of the descending fibers in the lateral corticospinal tract are uncrossed. These ipsilateral descending projections control musculature of the trunk and proximal limbs and thus participate in the maintenance of an upright stance and in gross positioning of the limbs.
The pyramidal tract has a somatotopic organization throughout its course. (The origin, termination, and function of this tract have been described more fully in Chapter 5.)
The pyramidal corticospinal and corticobulbar tracts act primarily as a system for controlling movement. However, they also contain axons that modulate the function of ascending systems in the thalamus (ventroposterior nucleus), brain stem (dorsal column nuclei), and spinal cord (dorsal horn laminas).
The Extrapyramidal Motor System
The extrapyramidal system is a set of subcortical circuits and pathways, phylogenetically older than the corticospinal system, which includes the corpus striatum (caudate nucleus, putamen, and globus pallidus) together with the subthalamic nucleus, substantia nigra, red nucleus, and brain stem reticular formation (Figs 13–2A, 13–4, and 13–5). Some authorities include descending spinal cord tracts other than the corticospinal tracts (such as the vestibulospinal, rubrospinal, tectospinal, and reticulospinal tracts) in the extrapyramidal motor system. Cortical and subcortical components of the motor system are richly interconnected, either directly and reciprocally, or by way of fiber loops. Many of these interconnections involve the extrapyramidal system, and the majority traverse the basal ganglia.
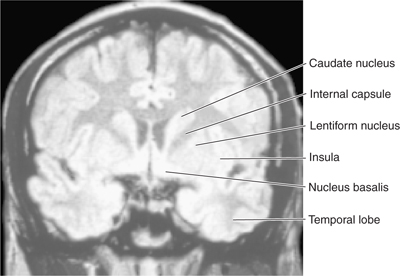
FIGURE 13–4 Magnetic resonance image of a coronal section through the head at the level of the lentiform nucleus.
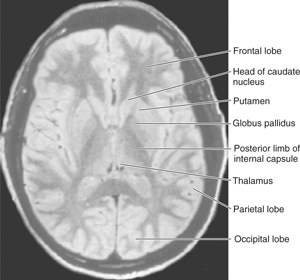
FIGURE 13–5 Magnetic resonance image of an axial section through the head at the level of the lentiform nucleus.
Basal Ganglia
Pathways and nuclei: The anatomy of the gray masses in the forebrain that make up the basal ganglia has been described in Chapter 10 (see Figs 10–17, 10–18, and 13–2). The striatum (caudate and putamen) is the major site of input to the basal ganglia (see Fig 13–2B). The striatum receives afferents via the corticostriate projections from a large portion of the cerebral cortex, especially the sensorimotor cortex (areas 4, 1, 2, and 3), the more anterior premotor cortex (area 6), and the frontal eye fields (area 8) in the frontal and parietal lobes. These corticostriate projections are excitatory. The striatum also receives inputs from the intralaminar thalamic nuclei, substantia nigra, amygdala, hippocampus and midbrain raphe nuclei. Many inhibitory (gamma-aminobutyric acid [GABA]-ergic) and a smaller number of excitatory interneurons (the latter in some cases using acetylcholine as a transmitter) are present within the striatum.
The caudate and putamen send inhibitory (GABA-ergic) axons to the inner part of globus pallidus (GPi), which is the major outflow nucleus of the basal ganglia. These projections provide a strong inhibitory input to the globus pallidus (see Fig 13–2C).
The globus pallidus, GPi (internal part) is one of the two major output nuclei of the basal ganglia. GPi sends inhibitory axons (GABA-ergic) to the ventral nuclei (ventral anterior, VA; and ventral lateral, VL) of the thalamus (which also receives input from the cerebellum, the subthalamic nucleus, and substantia nigra). Axons from the globus pallidus project to the thalamus by passing through or around the internal capsule. They then travel in small bundles (the ansa lenticularis and the lenticular fasciculus, also known as the H2 field of Forel) before entering the thalamus (see Fig 13–2C). The VA and VL thalamic nuclei complete the feedback circuit by sending axons back to the cerebral cortex (see Fig 13–2D). The circuit thus traverses, in order,
Cortex →striatum →globus pallidus (internal, GPi) →thalamus →cortex
Another important feedback loop involves the second major outflow nucleus of the basal ganglia, the substantia nigra, which is reciprocally connected with the putamen and caudate nucleus. Dopaminergic neurons in the pars compacta of the substantia nigra project to the striatum (the nigrostriatal projection), where they form inhibitory synapses on striatal neurons that have D2 dopamine receptors, and excitatory synapses on neurons that have D1 dopamine receptors (see Fig 13–2B). Reciprocal projections travel from the striatum to the substantia nigra (striatonigral projection) and are also inhibitory (see Fig 13–2C). This loop travels along the pathway
Cortex → striatum → substantia nigra → striatum
Neurons in the substantia nigra and GPi also send inhibitory projections to the thalamus (VA and VL), which, in turn, sends projections to the sensorimotor cortex. Substantia nigra (pars compacta) also sends modulatory projections (mesolimbic and mesocortical projections) to the limbic system and cortex. This pathway involves the following circuit:

The pars reticulata of the substantia nigra (SNr) receives input from the striatum, and sends axons outside the basal ganglia to modulate head and eye movements.
The subthalamic nucleus (also called the nucleus of Luys) also receives inhibitory inputs from the globus pallidus and from the cortex; efferents from the subthalamic nucleus return to the globus pallidus (see Fig 13–2C). Thus, the subthalamic nucleus participates in the feedback loop:
Cortex → globus pallidus → subthalamic nuclei → globus pallidus → cortex
Another loop involves the cerebellum. Portions of the thalamus project by way of the central tegmental tract to the inferior olivary nucleus; this nucleus, in turn, sends fibers to the contralateral cerebellar cortex. From the cerebellum, the loop to the thalamus is closed via the dentate and contralateral red nuclei.
Although there are no direct projections from the caudate nucleus, putamen, or globus pallidus to the spinal cord, the subthalamic region, including the prerubral field and the red nucleus, is an important relay and modifying station. Projections from the globus pallidus to the red nucleus converge with inputs from the motor cortex and the deep cerebellar nuclei. Efferent fibers from the red nucleus descend in the spinal cord as the rubrospinal tract, which modulates the tone of flexor muscles (see the following section).
The organizational theme for the basal ganglia involves complex loops of neurons (including many inhibitory neurons) feeding back to the sensorimotor cortex. These neuronal loops play an important role in motor control. Electrical engineers are well acquainted with abnormal oscillations, or “ringing,” that can occur when inhibitory feedback circuits are damaged. Disorders of the basal ganglia are often characterized by abnormal movements that can be repetitive or rhythmic.
The motor control circuits passing through the basal ganglia that are involved in movement disorders such as Parkinson’s disease have been conceptualized as operating in a manner summarized in FIGURE 13–6A. According to this model, excitatory synaptic output from the precentral and postcentral motor and sensory cortex is directed to the putamen. The putamen also receives projections from the pars compacta of the substantia nigra (SNc). Output from the putamen is directed toward the internal portion of the globus pallidus (GPi) and the pars reticulata of the substantia nigra (SNr) via two pathways (the direct and indirect pathways). Monosynaptic inhibitory projections from the putamen project via the direct pathway to GPi/SNr and tend to enhance motor activity. A series of polysynaptic connections extends from the putamen, within the indirect pathway, through portions of the external part of the globus pallidus (GPe) and the subthalamic nucleus (STN), with the net outcome of suppression of motor activity. In addition, mutual inhibitory connections exist between GPe and GPi/SNr. Outputs from GPi/SNr project toward the ventro-lateral nuclear group of the thalamus (VL), and the VL in turn projects back to the cortex. Importantly, most of the intrinsic connections within the basal ganglia, and the GPi/SNr projections, are inhibitory (GABA-ergic), except for the projection between STN and GPi/SNr. Changes in activity in this circuitry as a result of cell death in SNc (Fig 13–6B), which disturb the balance between enhancement and suppression of motor activity, are discussed later and have significant implications for Parkinson’s disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








