Square pulse commonly used for spinal cord stimulation, which is dependent on amplitude and pulse width
The pulse or stimulation rate can be manipulated to create distinct pulses. Settings of the SCS can vary between a low rate or a merging of pulse sensations with higher frequency stimulation. Lower frequencies result in a more distinct, slower pulse, while higher frequencies result in a more continuous, smoother sensation.
Complex mathematical modeling of the impact of these parameters on SCS function has been done. Named for Jan Holsheimer, the concept of mapping out the proper lead positioning and concomitant SCS parameters for optimal effect has become known as Holsheimer mapping [8]. While they are advanced concepts, the mathematical underpinnings of SCS programming are important issues to understand when complex programming is required. Pulse width provides an illustrative example of this concept. For example, if a spinal cord stimulator lead is placed in a more lateral position within the epidural space, a longer pulse width may activate the spinal cord nerve root and cause discomfort. In this scenario, narrowing the pulse width may be beneficial.
Electrical Field
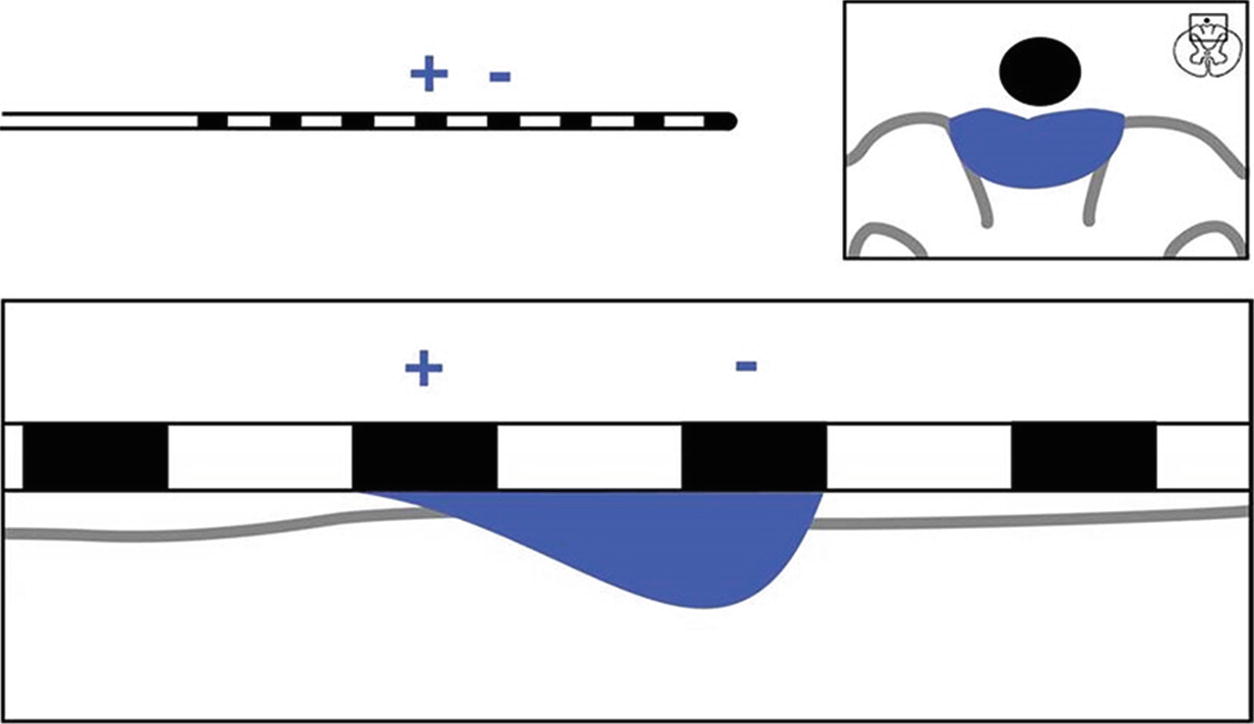
Single anode and cathode configuration
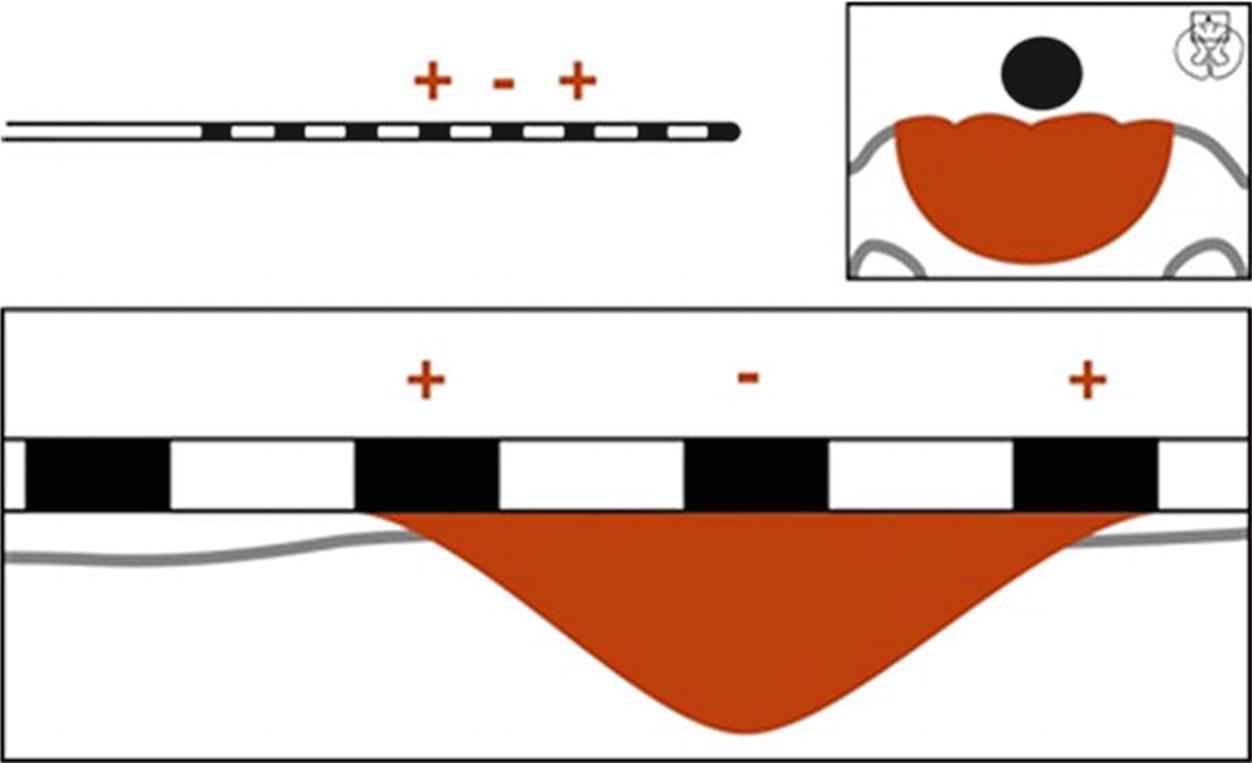
Guarded cathode configuration
Technical Aspects of Lead Placement
Preplacement Planning
Spinal cord stimulation can be utilized at all spinal levels and as such requires some preplacement planning. For example, cervical leads can be placed at the cervico-thoracic junction or via a lumbar access site with the lead maneuvered through the epidural space to the cervical target. Both approaches have merit but different applications. If one is conducting a temporary trial, then the “work” of threading leading leads from the lumbar spine for a patient who may not derive benefit may be futile [9]. Conversely, if the leads are for a permanent implant, the lumbar placement negates the need for lead extensions or extensive subcutaneous tunneling. Similarly, if leads are to be placed in the sacral space, a decision must be made whether to attempt placement in a retrograde fashion or via the sacral hiatus.
While there is wide variability among individual patients, there are some guidelines with regard to lead placement targets which may assist the clinician in preplacement planning. For instance, it is widely accepted that in the cervical spine, the C2–C5 region will encompass the shoulder to the arm/hand. Likewise many have observed that obtaining paresthesia coverage for pain in the cervical axial spine is often difficult. Pain of thoracic origin can be broadly categorized as intercostal and visceral. Intercostal paresthesia can often be obtained at or just above the thoracic level of injury in a lateral position, while visceral pain (an area of emerging application for SCS) is currently not well defined and can be highly variable when obtained at all. Paresthesia coverage of pain of lumbar origin is better described. Classic teaching states that the “target zone” for most lumbar pain has an upper limit at T8 level with neurologic mapping undertaken to find the exact location between T8 and L1 that works best for a given patient. Lumbar lead placement between L2 (termination of the spinal cord) and L5 is occasionally helpful and has many features in common with nerve root stimulation since the dorsal horn terminates at the T12–L1 level with the conus medullaris (the distal portion of the spinal cord proper at the L1–2 level). Sacral targets, though technically difficult to access, typically are relatively straightforward in their preplacement assessment in that the affected painful level is typically the optimal site for lead placement.
Physiologic Versus Anatomic Positioning
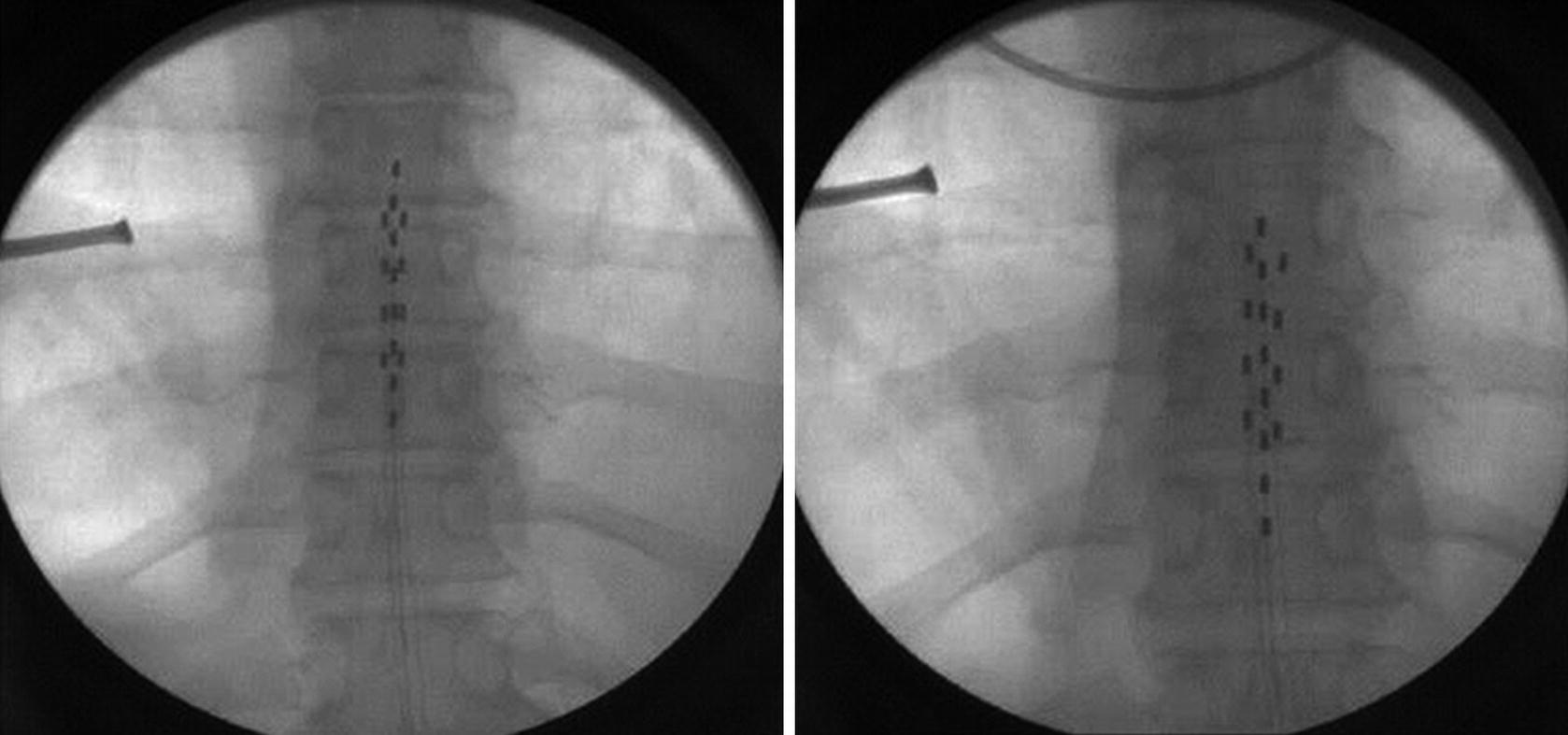
Fluoroscopic image obtained during initial placement (left panel) of the “ideal” lead placement and the physiologically correct placement for this particular patient (right panel)
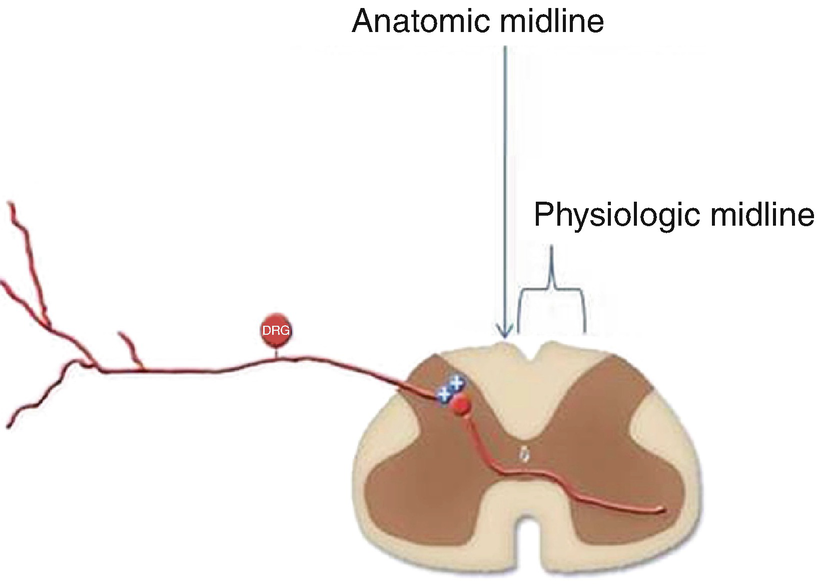
The anatomical midline and the physiological midline or “sweet spot ,” terms that describe the consistent finding that ideal anatomic position of the SCS lead under imaging (anatomic midline) often requires repositioning of the lead to less aesthetically pleasing but more desirable physiologic position to obtain paresthesia coverage of the painful area (physiologic midline)
Anatomical Conservations
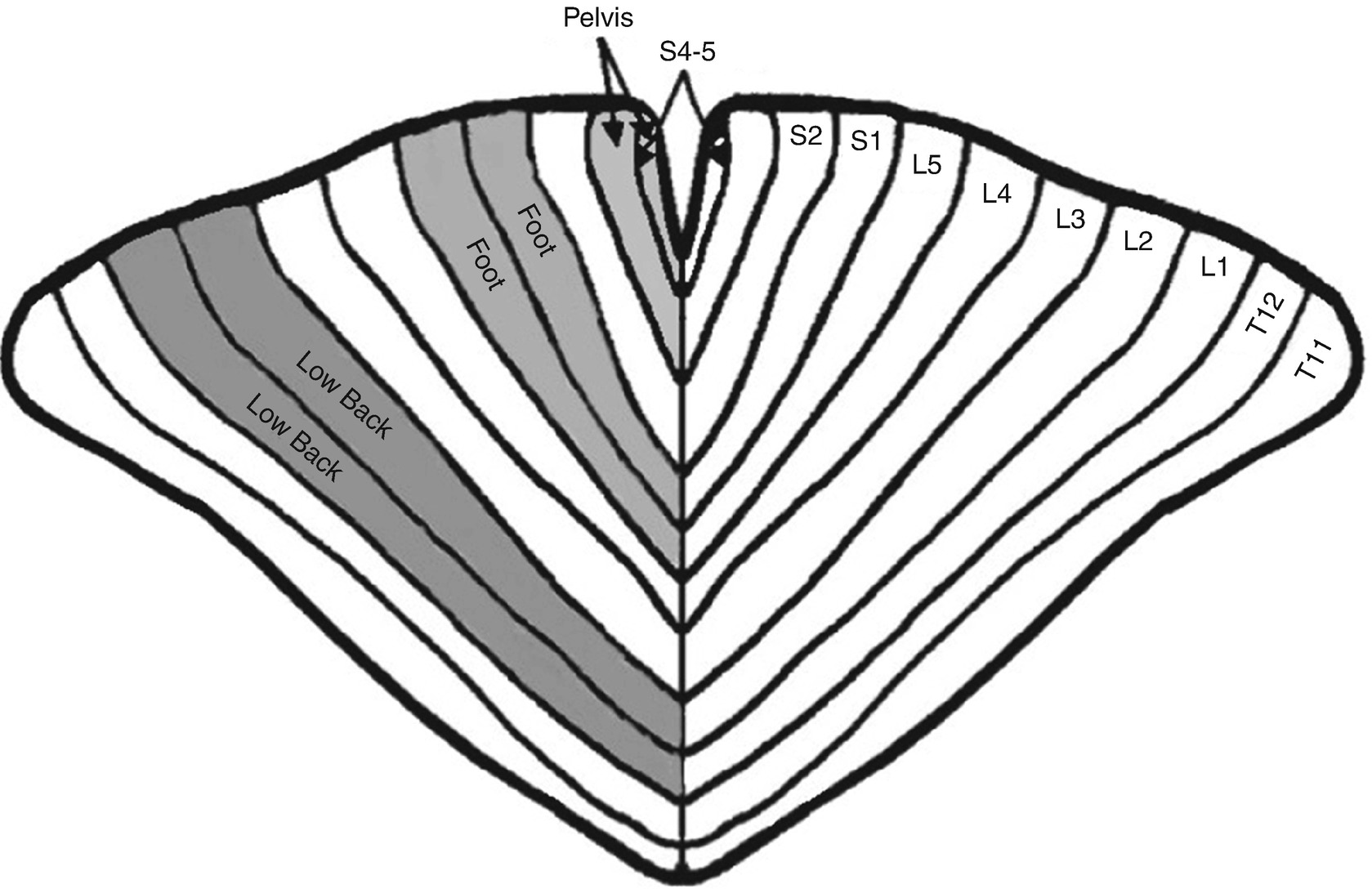
Nerve fibers of more distal structures are contained in more central locations within the spinal cord. These fibers become more superficial as they near the exit point within the spinal cord. A spinal cord homunculus analogous to the homunculus at motor cortex has been described that suggests that sacral, lumbar, and thoracic fibers occupy fixed positions within the spinal cord ranging from medial to lateral, respectively
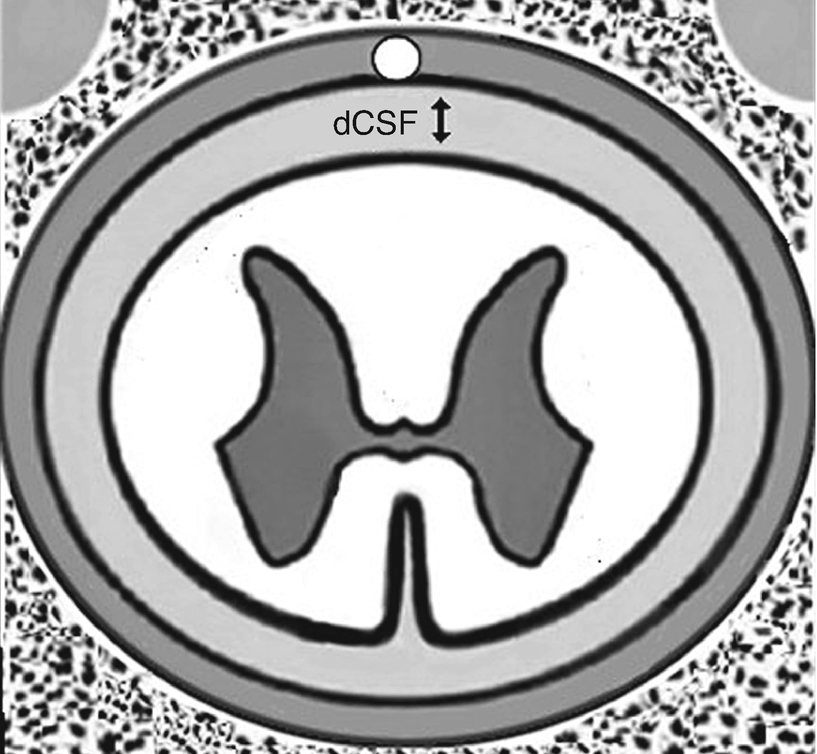
The CSF thickness varies along the spinal column and can significantly impact stimulation
Technical Considerations and Trialing Techniques
Technical Considerations
The number of contacts and leads to be utilized in SCS treatment is a matter of much conjecture and little conclusive evidence. In the mid-2000s, a single or dual four-contact lead system was the state of the art. A study conducted during this period suggested that there was little advantage in adding a second lead for either radicular lower extremity or low back pain [12]. In this study, the dual lead system was associated with faster implantable pulse generator (IPG) discharge, without significant improvement in perceived pain relief. Technological advances in IPG battery life coupled with more sophisticated programming options have led to rapid adoption of eight-contact leads which when used in an 8 × 2 array result in all channels of the IPG occupied and available to be utilized [13]. A 16-contact lead has recently entered the market and is already undergoing clinical testing using a 16 × 2 array for enhanced coverage and reducing the need for lead adjustment due to lead migration. The enhanced coverage would only necessitate reprogramming as opposed to additional surgeries.
Leads configured in multiple combinations such as two leads (bipole) and three leads (tripole) have been suggested to enhance coverage of low back pain. This concept is currently under investigation. The introduction of the tripole concept allows the clinician to mimic lead contact coverage obtained with a surgical plate or “paddle” lead [14]. The broad “paddle” lead has wider contact spacing allowing coverage of a wider area within the spinal cord. There also seems to be less lead migration with the paddle lead.
Another advantage over the percutaneous lead lies in the shape of the lead itself. The cylindrical percutaneous lead “radiates” an electrical field in a 360° direction, while the surgical paddle lead directs current toward the spinal cord. It has been proposed that this arrangement directs current “deeper” into the spinal cord and may allow better axial back pain coverage. With the multiple contact percutaneous lead, the greater contact capability (8 × 1) does allow the clinician to potentially retain paresthesia coverage even if small degrees of lead migration occur [15]. It remains to be seen if the increased number of contact points is of significant benefit from a clinical perspective.
Interleaving
The programming capabilities of multiple contact points allow the programmer to utilize an advanced concept known as interleaving to cover multiple areas of pain. The fundamental basis of this approach utilizes the programming of the IPG to rapidly (in microseconds) switch back and forth between programs on separate portions of the lead that cover different areas of pain. For example, in an 8 × 2 configuration, lead contact 0–4 on a left-sided lead may cover low back pain, while 11–15 on the right may cover the radicular lower extremity pain. With rapid cycling between the two areas of lead contact, the patient perceives coverage of both areas. The interested reader is directed to several excellent manuscripts on this topic.
Constant Voltage Versus Constant Current
As discussed previously, all SCS systems are bound by Ohm’s law in the way that they transduce the electrical signal to the biological system. If resistance (impedance in these alternating current systems) is relatively constant, and this is dependent upon the biological milieu, the only variables that can be manipulated are voltage and current. The advantages of both approaches can be theoretically debated with excellent arguments emerging for both types of systems. One study has compared constant voltage and constant current in a randomized trial, allowing the patient to determine whether there was a preference between constant voltage and constant current systems. In this small preliminary study, patients could not reproducibly identify constant current systems from constant voltage systems, suggesting that the theoretical differences may not translate into clinically meaningful differences in therapy [16]. This fascinating topic deserves further research.
Spinal Cord Stimulation Trial Techniques
After careful preplacement planning has been accomplished, it is necessary to plan the trailing process. It is recommended that all patient candidates for SCS should undergo a pretrial psychological assessment to determine if there are unrealistic expectations, secondary gain issues, psychological issues that have not been maximally explored and treated, or other biopsychosocial factors that may impact treatment success. Once this has been done, it is necessary to discuss with the patient the trialing technique. The purpose of the trial is to temporarily allow the patient to experience the sensation of SCS without having to endure the full implantation process with the IPG. There are two types of percutaneous spinal cord stimulator trials: (1) temporary percutaneous and (2) staged percutaneous placement with permanent anchoring of the leads. Each trialing method has advantages and disadvantages. The more common temporary percutaneous method entails securing the trialed lead to the skin with suture or other easily reversible material in a fashion that is quickly and simply removed. The percutaneous placement with permanent anchoring method requires surgical incision after lead placement and anchoring identical to that which is done with permanent implantation. The anchored leads are then connected to disposable trial connectors and exteriorized via tunneling in an operative setting.
The advantages of the more common temporary percutaneous placement in comparison to permanent anchoring method are (1) easy placement and removal, (2) can be done in office procedure setting (whereas the surgical anchoring requires a traditional operating suite), (3) less post-procedure discomfort to distract the patient from the trial process, and (4) less invasive. Conversely, the percutaneous placement with permanent anchoring results in a more accurate trial to implant experience and less surgical time required for implantation of the IPG [17]. Additionally, the IPG placement can be performed under deeper sedation/general anesthesia since sensory mapping is not necessary. Occasionally, the results of the temporary trial are superior to the actual implant using the former method resulting in significant patient dissatisfaction. In the pretrial planning process, if it is suspected that spinal epidural access or lead manipulation will be difficult, it may be reasonable to do the staged trial with permanent anchoring; otherwise, most centers utilized the temporary percutaneous method.
Regardless of trialing method, it is imperative that adequate time with the therapy be given to the patient to determine efficacy. Balancing the need for time with the therapy with the risk of infection usually results in 3–5-day trial period although some clinicians advocate for at least 7 days [18]. Experience with infection rates of epidural catheters suggests that any trial up to 7–10 represents low risk from an infection standpoint. During the trial, evaluation of functional capacity, sleep hygiene, and pain reduction is key. The person who does not derive functional benefit but claims pain relief should be evaluated closely.
High-Frequency Spinal Cord Stimulation and High-Frequency Burst Stimulation
For decades traditional SCS settings have dominated the market; however, over the last 8 years, there has been promising data emerging from the increasing use of high-frequency (10 kHz) spinal cord stimulation devices (HF10) burst stimulation. These devices have similar clinical indications as the traditional SCSs and have been approved by the US Food and Drug Administration for the treatment of both back and leg pain. They operate based on the same positional and electrical current delivery models as the traditional devices. However, contrary to the frequencies used for traditional SCS (40–120 Hz), these newer devices create currents with frequencies of 10 kHz or use burst impulses of five separated at 40 Hz with internal burst frequencies of 500 Hz or greater. While the frequencies of these devices are increased, the amplitude of the current itself is markedly decreased to levels such that they don’t elicit a motor or sensory response. Therefore, in contrast to the previously described devices so highly reliant on paresthesia development to determine both proper placement and efficacy, high-frequency devices at appropriate settings do not require what can be unformatable paresthesias to generate a response.
While these devices are still in their infancy, the data are promising. A 2-year multicenter randomized control trial which included nearly two hundred patients demonstrated long-term superiority of HF10 therapy when compared to the use of traditional SCS [19]. The study examined 198 patients with chronic leg and back pain randomized into HF10 therapy or traditional SCS treatment groups and used primary and secondary end points of 3-, 12-, and 24-month intervals with respect to pain relief. What was found is that at each temporal interval, the patients demonstrated both non-inferiority and superiority of pain relief in the HF10 population [19].
In response to the significant clinical benefit of these devices, researchers have begun to explore and postulate regarding their mechanism of action. The Gate Control Theory which lead to the development of the initial SCS treatment devices does not hold water with regard to HF10 devices. The pillar of traditional paresthesia-based SCS devices is the activation of the dorsal columns and gracile nucleus to alter the interpretation pathways of pain [20]. Multiple animal studies and computer modeling have shown that HF10 therapy does not activate or even change the conduction properties of the dorsal column fibers, the gracile nucleus, or even simple peripheral mechanical stimulation responses [19, 20]. The response to the device itself is described vastly different: HF10 treatment requires hours to days to develop maximum pain relief, whereas SCS relief is apparent nearly immediately. Tiede et al. performed a multicenter prospective trial of HF10 therapy on patients who had previously tried and failed traditional SCS therapy and found 88% of the patients responded to the HF10 treatment [20]. This study further alludes to a unique mechanism of action of the HF10 treatments given its marked ability to induce relief in patients who were otherwise nonresponders to treatment.
There are multiple “working hypothesis” undergoing investigation currently as to how HF10 therapy mitigates these pain pathways: depolarization blockade, membrane integration, desynchronization, and glial-neuronal interaction [20]. The depolarization blockade theory suggests that an electrical field is created similar to the tradition model such that the neurons are further depolarized but in a local revisable manner. The membrane integration hypothesizes that the summation of the pulsatile signals creates an action potential whereas the single pulses alone would not induce a response. Both the depolarization and the membrane integration theories which mandate an altered neuronal stimulation response have data to contradict the theories. The desynchronization theory describes complex neuronal networks firing in synchrony to communicate pain and HF10’s ability to desynchronize these firings. Finally, although the published data are lacking, the glial-neuronal theory describes a reformed activation of the astrocytes and microglia cells to alter the somatosensory pathways of pain [20].
Clinical Indications
- 1.
Failed back surgery syndrome : It has been shown that SCS has better outcomes than reoperation. These findings suggest that a trial of SCS before considering a second back surgery should be a part of the treatment algorithm.
- 2.
Radicular pain : Pain of radicular nature in a classic dermatomal distribution in either the cervical, thoracic, or lumbar spine has a relatively strong evidence base suggesting efficacy.
- 3.
Neuropathic pain : Perhaps the strongest indication is the intense pain of neuropathic origin. Entities such as complex regional pain syndrome types 1 and 2, post-herpetic neuralgia, and post-amputation limb pain all respond well to SCS. Of these indications, CRPS has strong clinical data to suggest efficacy.
- 4.
Peripheral vascular disease : Such as Raynaud’s phenomena, nonoperative limb ischemia, chronic angina, and Berger’s disease.
- 1.
Visceral/abdominal pain : There are case studies to suggest that neuromodulation can successfully be used to improve analgesia for pancreatitis and other pain of visceral origin.
- 2.
Peripheral neuralgia : Spinal cord stimulation technology has been successfully used to treat peripheral nerve pain such as ilioinguinal/iliohypogastric neuralgia and occipital neuralgia.
- 3.
Peripheral field nerve stimulation (PFNS) : While still in the emerging stages, there is evidence of improvement with pain of myofascial and other origins that is resistant to treatment with subcutaneously placed electrodes. There have been studies published that discuss a cross-talk between the epidural and peripherally placed electrodes providing a synergistic effect for resistant peripheral pain syndromes.
Of these applications, peripheral nerve stimulation has strong data to suggest its efficacy, while visceral/abdominal applications and PFNS are still in the early stages of description.
Complications
Complications summary
N | Complication rate (%) | |
|---|---|---|
All complications | 81 | 34.6 |
Hardware | 60 | 25.6 |
IPG discomfort or migration | 26 | 11.1 |
Lead migration | 20 | 8.5 |
Lead malfunction or fracture | 10 | 4.3 |
IPG malfunction | 4 | 1.7 |
Biologic | 21 | 9.0 |
Infection | 10 | 4.3 |
Unwanted paresthesia or dysesthesia | 6 | 2.6 |
Dehiscence or seroma of wound | 5 | 2.1 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







