Chapter 2 Cranial Anatomy
Nothing is more fascinating and has more layers of potential knowledge than the anatomy of the central nervous system (CNS). Like the microcircuits of a computer, each portion of the CNS interconnects to another. We deal with the development of the brain in Chapter 9, so we address only the pertinent aspects of normal anatomy in this chapter. Our approach in this chapter is to introduce the basic morphologic building blocks of the CNS in the first section, followed by the functional units in the latter section. (Function follows form.)
TOPOGRAPHIC ANATOMY
Cerebral Hemispheres
The main named areas of the frontal lobe are the precentral gyrus (the primary motor strip of the cerebral cortex) and the three frontal gyri anterior to the motor strip: the superior, middle, and inferior frontal gyri (Fig. 2-1). On the medial surface one finds the cingulate gyrus just superior to and bounding the corpus callosum, and the gyrus rectus extending along the medial basal surface of the anterior cranial fossa (Fig. 2-2).
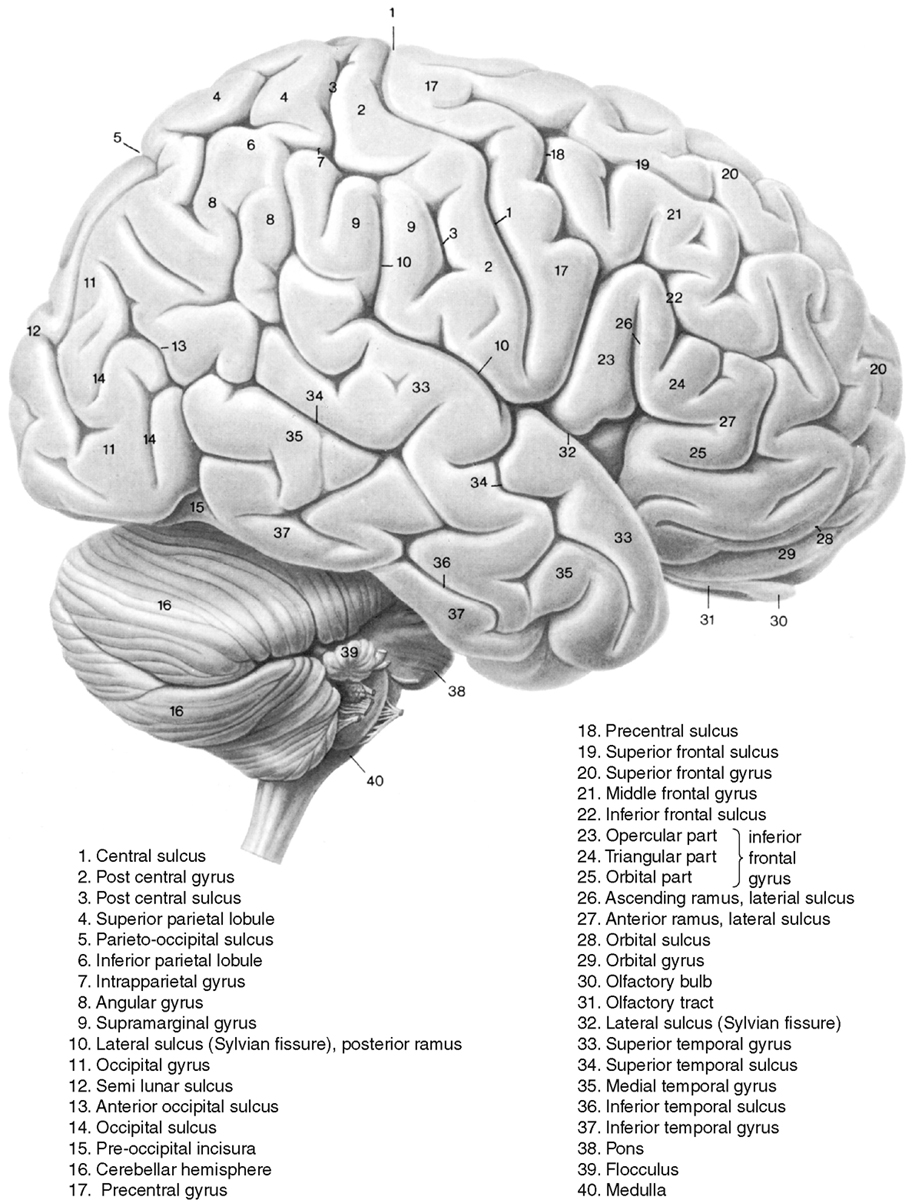
Figure 2-1 Surface anatomy of the brain from a lateral view. Gyri are labeled in this figure.
From Nieuwenhuys R, Voogd J, van Huijen C: The human central nervous system: a synopsis and atlas, rev ed 1, Berlin, Springer-Verlag, 1988.
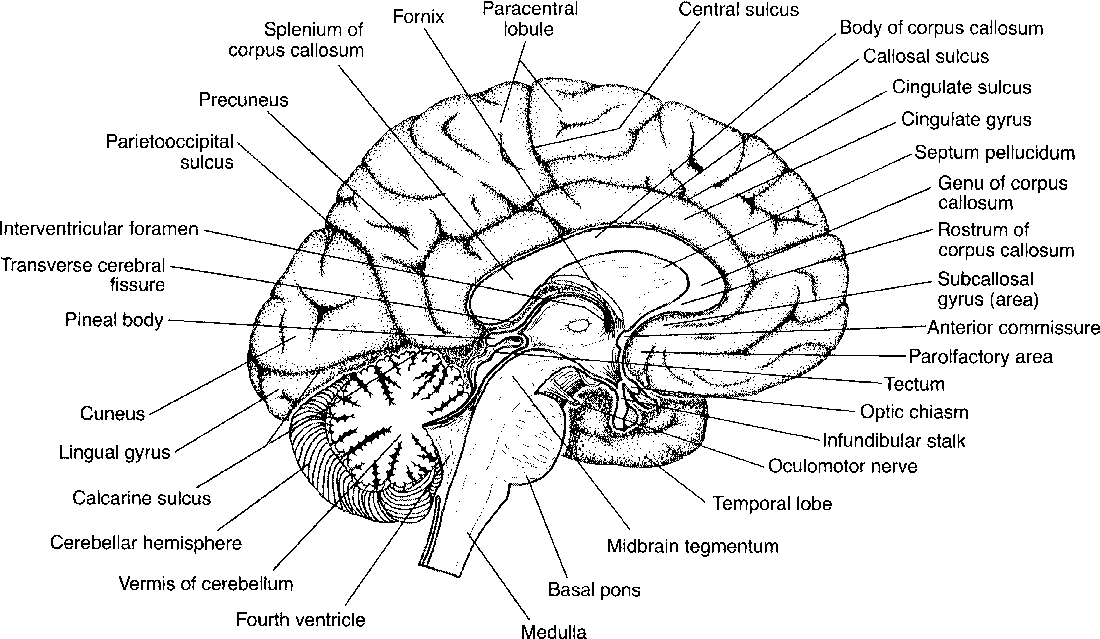
Figure 2-2 Medial surface of brain. Midsagittal view of the brain.
From Burt AM: Textbook of neuroanatomy. Philadelphia, WB Saunders, 1993, p. 159.
The parietal lobe contains the postcentral gyrus (the center for somatic sensation), the supramarginal gyrus just above the temporal lobe, and the angular gyrus near the apex of the temporal lobe (see Fig. 2-1). Two superficial gyri of note are the superior and inferior parietal lobules, which are separated by an interparietal sulcus. On its medial side the precuneate gyrus is present in front of the parieto-occipital fissure, with the cuneate gyrus posteriorly in the occipital lobe (see Fig. 2-2).
The temporal lobe contains the brain-functioning elements of speech, memory, and hearing. Superior (auditory), middle, and inferior temporal gyri are seen on the superficial aspect of the brain (see Fig. 2-1). Deep to the sylvian fissure is the insula, or isle of Reil, which is bounded laterally by the opercular regions. The frontal operculum (superior to the sylvian fissure and in the frontal lobe) contains portions of Broca’s motor speech area. The inferior part of the insula near the sylvian fissure is called the limen of the insula. The inferior and medial surface of the temporal lobe reveals the parahippocampal gyrus with the hippocampus just superior to it (Fig. 2-3). Anteriorly, the almond-shaped amygdala dominates. If you walked along the cortex of the parahippocampal-hippocampal region of the inferomedial temporal lobe, you would get dizzy because it simulates a spiral staircase. But if you started at the right collateral sulcus (in the coronal plane) just inferior to the parahippocampus and traveled northward, you would first hit the entorhinal cortex, then turn at the parasubiculum, pass along the subiculum proper, and continue laterally to the presubiculum. All of these represent parahippocampal structures. You would then curl in a spiral into the hippocampus’ cornu ammonis and dentate gyrus with the fimbria found superomedially and the alveus on top of the cornu ammonis. The cornu itself has four zones of granular cells: CA1 (Sommer sector) is lateral, CA2 (dorsal resistant zone) is superior, CA3 (resistant Spielmeyer sector) is superomedial, and CA4 (end folium) is inferomedial. CA1 is also called the vulnerable sector because it is the most sensitive area of the brain (with the globus pallidus) to anoxia. CA3 is resistant to anoxic damage. Sclerosis of CA1 is the etiology of mesial temporal sclerosis or hippocampal atrophy and has been linked to febrile seizures.
The occipital lobe is the lobe most commonly associated with visual function. At its apex is the calcarine sulcus, with the cuneate gyrus just above it (posteroinferior to the parieto-occipital fissure) and the calcarine gyrus just below it (see Figs. 2-1 and 2-2).
Brain Stem
The mesencephalon differentiates into the midbrain. The midbrain is the site of origin of the third and fourth cranial nerves (CN III and IV). Additionally, the midbrain contains the red nucleus, substantia nigra, and cerebral aqueduct, or aqueduct of Sylvius (Fig. 2-4). White matter tracts conducting the motor and sensory commands pass through the midbrain. The midbrain is also separated into the tegmentum and tectum, which refer to portions of the midbrain anterior and posterior to the cerebral aqueduct, respectively. The tectum, or roof, consists of the quadrigeminal plate (corpora quadrigemina), which houses the superior and inferior colliculi. The tegmentum contains the fiber tracts, red nuclei, nuclei of cranial nerves III and IV, and periaqueductal gray matter. The substantia nigra is the anterior border of the tegmentum. Anterior to the tegmentum are the cerebral peduncles.
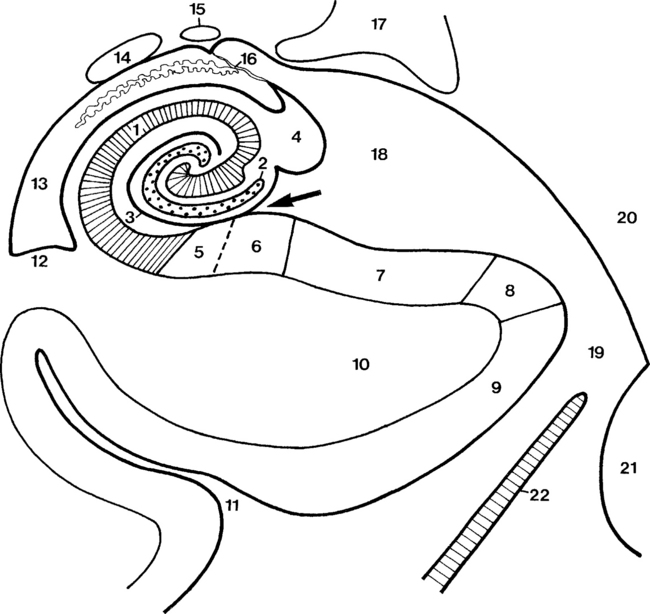
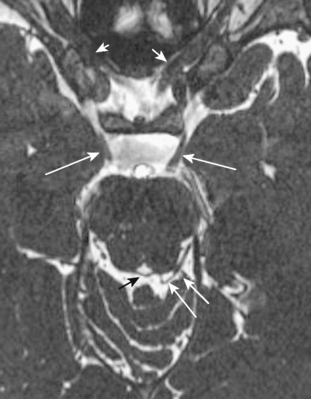
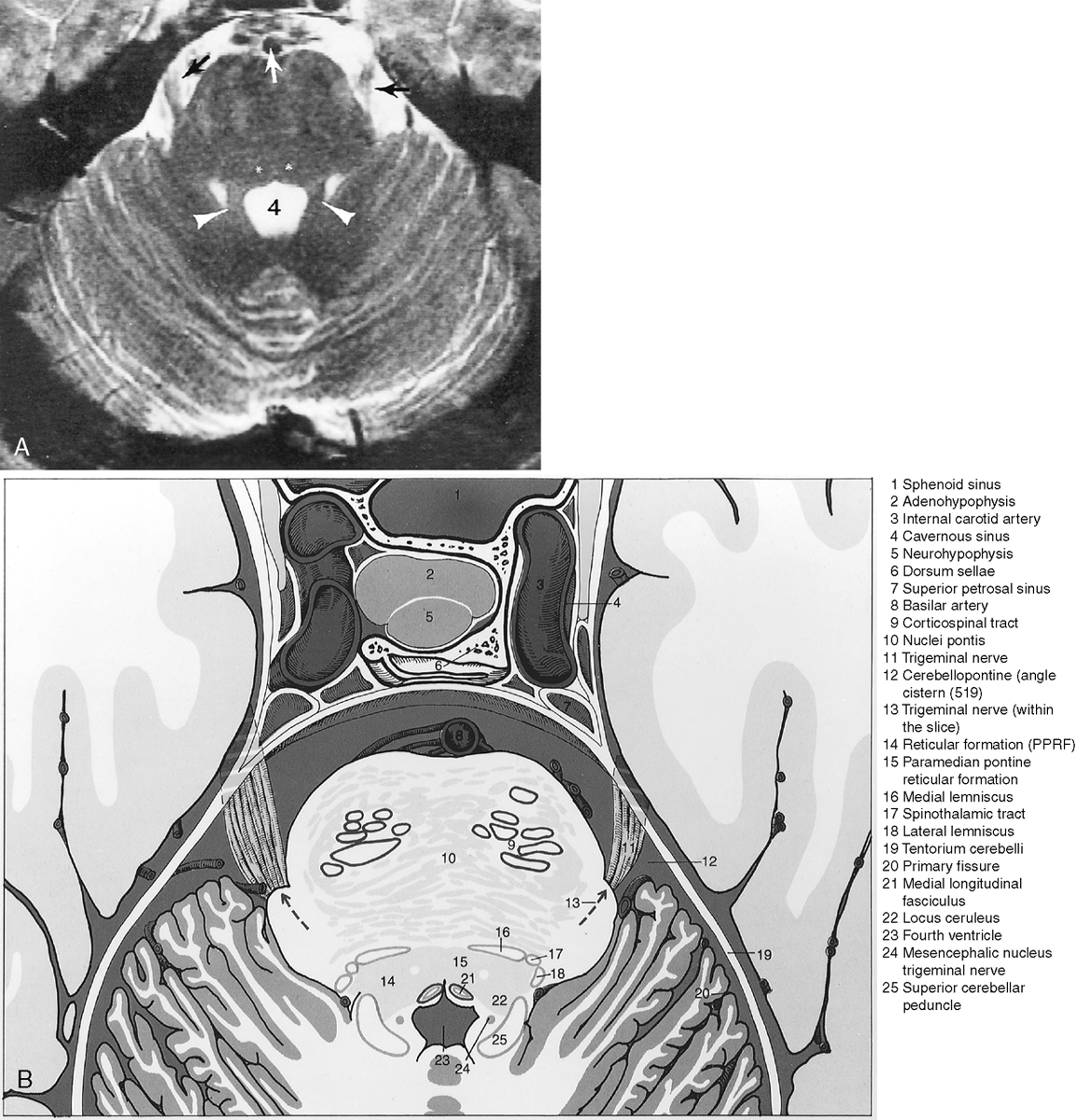
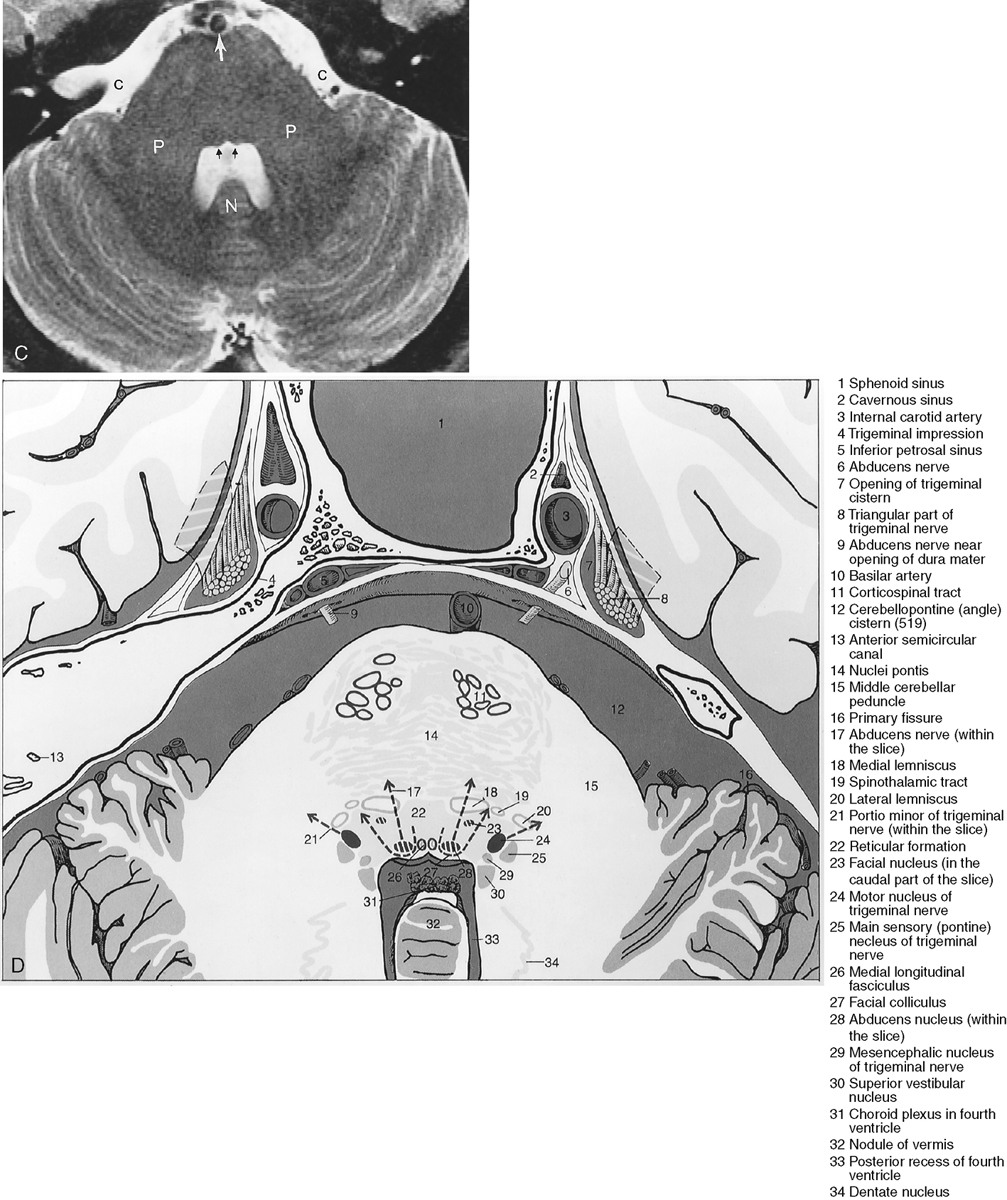
The metencephalon develops into the pons and cerebellum (see following section). The pons contains the nuclei for cranial nerves V, VI, VII, and VIII (Figs. 2-5 and 2-6). Pontine white matter tracts transmit sensory and motor fibers to the face and body (see Fig. 2-5). The pons also houses major connections of the reticular activating system for vital functions. One identifies the pons on the sagittal scan by its “pregnant belly.”
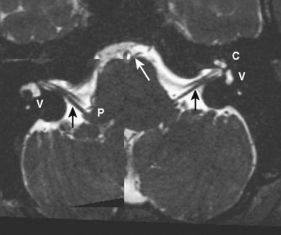
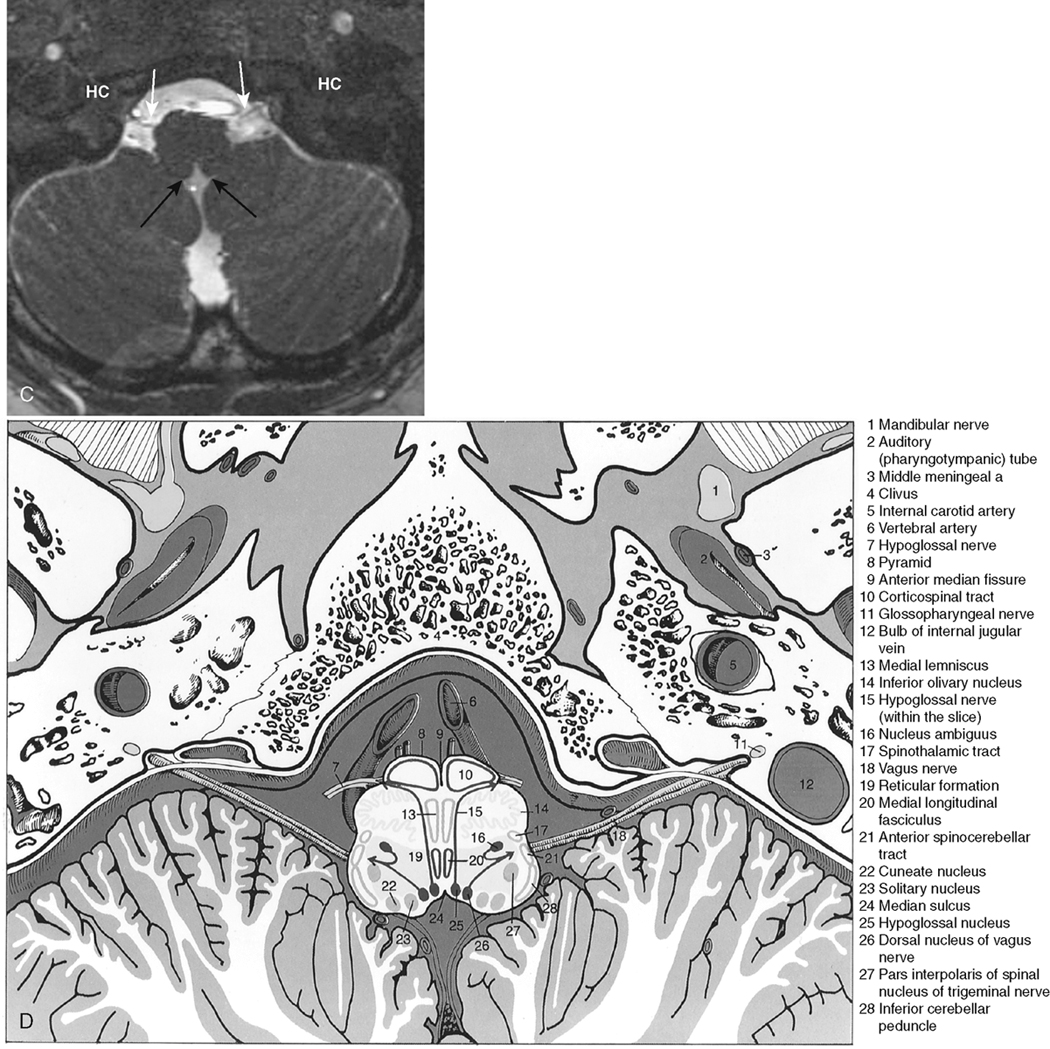
The myelencephalon becomes the medulla. The medulla contains the nuclei for cranial nerves IX, X, XI, and XII. Again, the sensory and motor tracts to and from the face and brain are transmitted through the medulla. Other named portions of the medulla include the pyramids, an anterior paramedian collection of fibers transmitting motor function, and the olivary nucleus in the midmedulla (Fig. 2-7).
Cerebellum
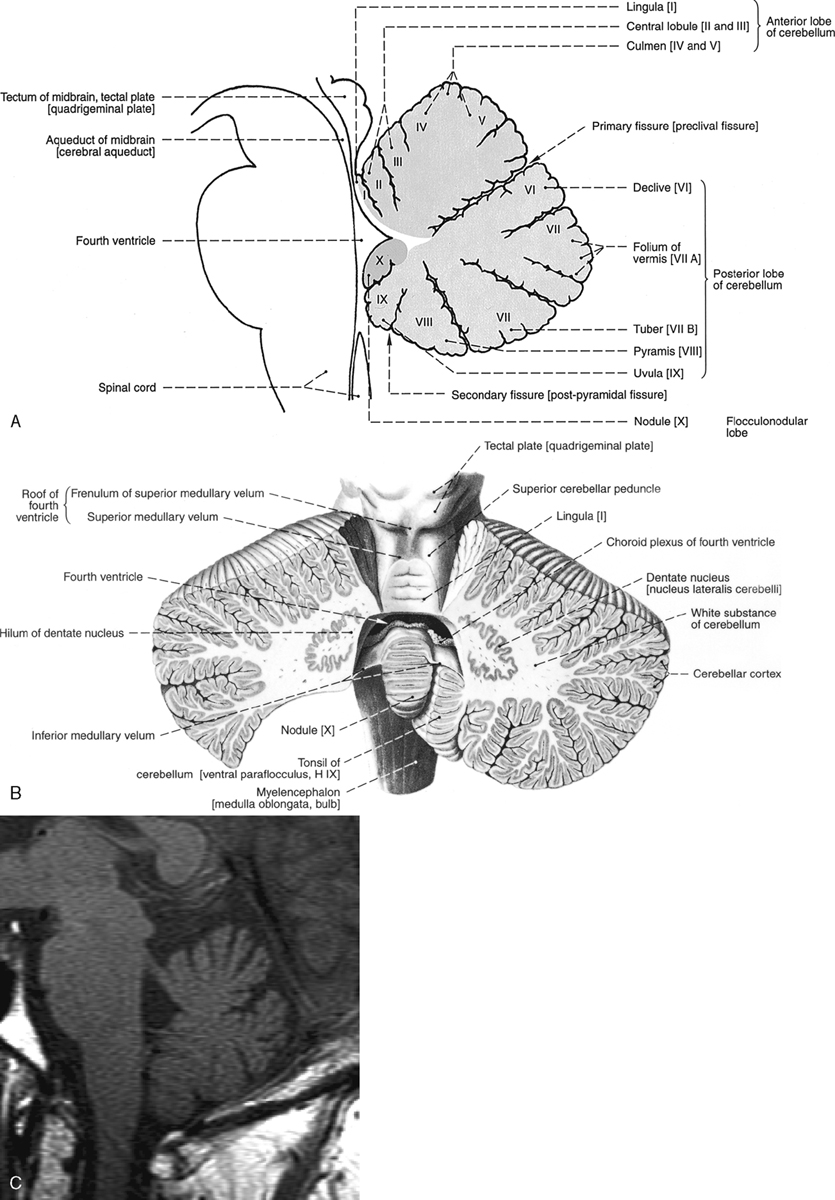
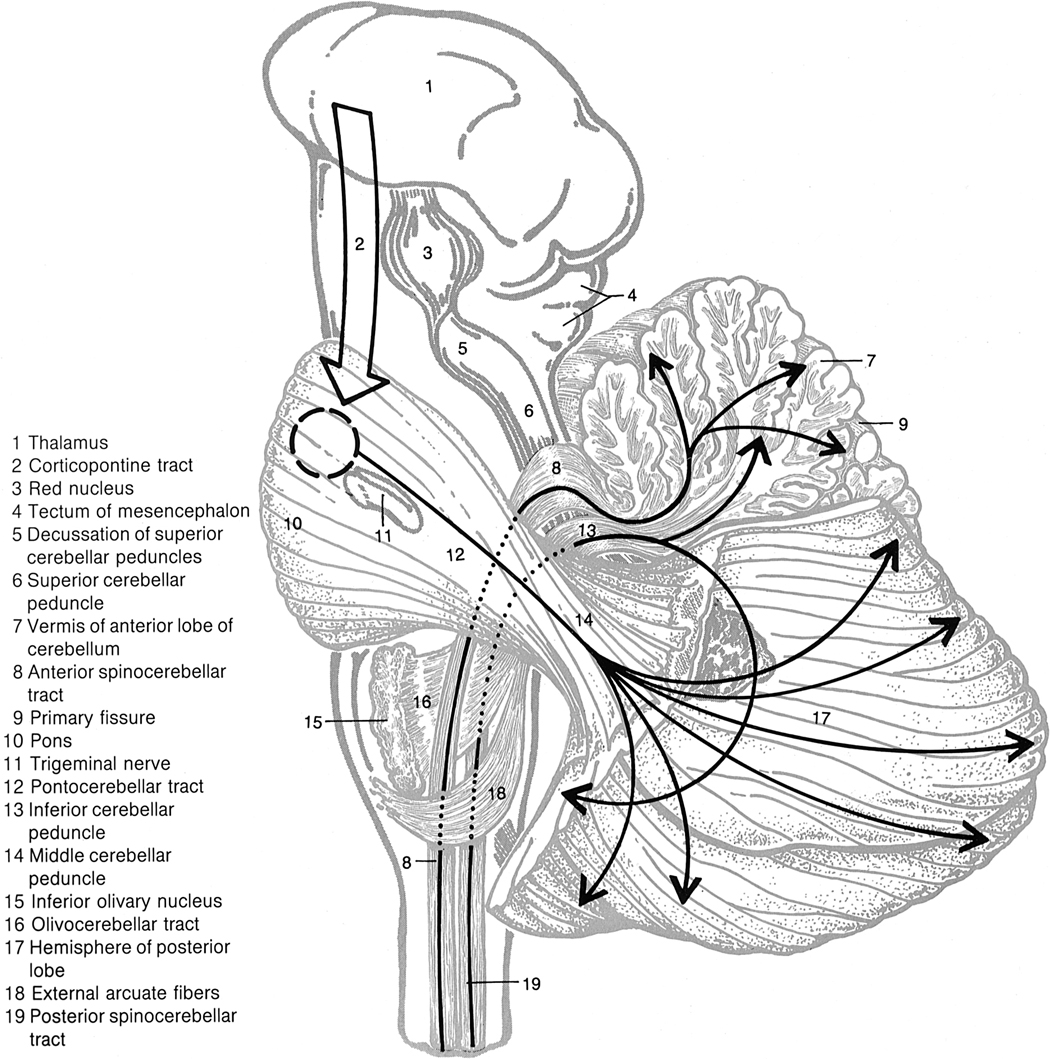
The cerebellum is located in the infratentorial space posterior to the brain stem. The anatomy of the cerebellum is complex, with many named areas. For simplicity’s sake, most people separate the cerebellum into the superior and inferior vermis and reserve the term cerebellar hemispheres for the rest of the lateral and central portions of the cerebellum. For those interested in details, the superior vermis has a central lobule and lingula visible anteriorly, and the inferior vermis has a nodulus, uvula, pyramid, and tuber on its inferior surface (Fig. 2-8). The superior surface provides a view of the culmen, declive, and folium of the superior vermis. Superolaterally, there is a bump called the flocculus, which may extend toward the cerebellopontine angle cistern. This is a potential “pseudotumor,” often misidentified as a vestibular schwannoma. The tonsils are located inferolaterally and are the structures that herniate downward through the foramen magnum in Chiari malformations.
Three major white matter tracts connect the cerebellum to the brain stem (Fig. 2-9). The superior cerebellar peduncle (brachium conjunctivum) connects midbrain structures to the cerebellum, the middle cerebellar peduncle connects the pons to the cerebellum, and the inferior cerebellar peduncle (restiform body) connects the medulla to the cerebellum.
Corpus Callosum
The medial surface of the brain in the midline is dominated by the corpus callosum. This is the large white matter tract that spans the two hemispheres. Its named parts include the rostrum (its tapered anteroinferior portion just above the anterior commissure), the genu (the anterior wide sweep over the third ventricle), the body or trunk (the superiormost aspect), and the splenium (the posteriormost aspect) (see Fig. 2-2). Embryologically, the genu and body form first, then comes the splenium, and finally the rostrum. This is useful in understanding partial agenesis of the corpus callosum.
Deep Gray Nuclei
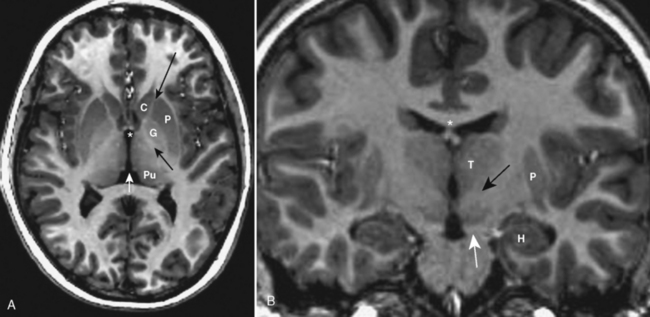
The basal ganglia are known by a number of names in the neuroanatomic literature. These gray matter structures lie between the insula and midline. The globus pallidus is the medial gray matter structure identified just lateral to the genu of the internal capsule (Fig. 2-10). Superficial to it lies the putamen. The caudate nucleus head indents the frontal horns of the lateral ventricle and is anterior to the globus pallidus; however, the body of the caudate courses over the globus pallidus, paralleling the lateral ventricle and ending in a tail of tissue near the amygdala.
Ventricular System
The flow of CSF runs from the choroid plexi in the floor of the lateral ventricles via the foramina of Monro, to the third ventricle, out the cerebral aqueduct of Sylvius, and into the fourth ventricle (Fig. 2-11). Each ventricle’s choroid plexus contributes to CSF production. After leaving the foramina of Magendie (medially) and Luschka (laterally) of the fourth ventricle, CSF flows into the cisterns of the brain and the cervical subarachnoid space and then down the intrathecal spinal compartment. The CSF ultimately percolates back up over the convexities of the hemispheres, where it is resorbed by the arachnoid villi into the intravascular space.
There are several named cisterns around the brain. The names, locations, and the structures traversing these cisterns are summarized in Table 2-1.
Table 2-1 Cisterns of the Brain
| Name | Location | Structures Traversing Cistern |
|---|---|---|
| Cisterna magna | Posteroinferior to fourth ventricle | None important |
| Circummedullary cistern | Around medulla | Posterior inferior cerebellar artery |
| Superior cerebellar cistern | Above cerebellum | Basal vein of Rosenthal, vein of Galen |
| Prepontine cistern | Anterior to pons | Basilar artery, cranial nerves V and VI |
| Cerebellopontine angle cistern | Between pons and porus acousticus | Anterior inferior cerebellar artery, cranial nerves VII and VIII |
| Interpeduncular cistern | Between cerebral peduncles anterior to midbrain | Cranial nerve III |
| Ambient (crural) cistern | Around midbrain | Cranial nerve IV |
| Quadrigeminal plate cistern | Behind midbrain | None important |
| Suprasellar cistern | Above pituitary | Optic chiasm, cranial nerves III and IV, carotid arteries, pituitary stalk |
| Retropulvinar cistern (wings of ambient cistern) | Behind thalamus | Posterolateral choroidal artery |
| Cistern of lamina terminalis | Anterior to lamina terminalis, anterior commissure | Anterior cerebral artery |
| Cistern of velum interpositum | Above third ventricle | Internal cerebral vein, vein of Galen |
| Cistern of the anterior cerebral artery | Above corpus callosum | Anterior cerebral artery |
FUNCTIONAL ANATOMY
Brodmann Areas
Although this is by no means an exhaustive review, Table 2-2 will help you define structure and function in Brodmann’s terms.
Table 2-2 Functional Anatomy by Brodmann Areas
| Brodmann Area # (alias) | Location | Function |
|---|---|---|
| Postcentral gyrus, paracentral lobule, parietal lobe | Primary somatosensory, rapidly adapting skin sensors, position sense | |
| 2 | As above, posterior to 1 | As above, proprioception from joints |
| As above, anterior to 1 | As above, fine tactile receptors in b, stretch receptors in a | |
| Precentral gyrus, frontal lobe | Primary motor | |
| Superior parietal lobule, posterior to 2 | Somatosensory association area, gross sensory areas | |
| Superior and middle frontal gyrus, anterior to 4; has lateral and medial surface parts | Premotor area, supplemental motor area, word retrieval, hand movement, stuttering, programming movements | |
| 7 | Superior parietal lobule, posterior to 5, some of precuneus | Counting, mathematics, somatosensory association, mental rotation |
| Anterior superomedial frontal gyrus, superior frontal gyrus | Frontal eye fields, mental state assessment, spatial attention and orientation | |
| 9 | Orbitofrontal, prefrontal, frontal gyri | Emotions, pain, motor association, intelligence |
| 10 | As above, anterior and inferior to 9; frontopolar region | Emotions, pain, higher intelligence, motor association |
| 11 | Gyrus rectus, inferior pole frontal lobe, orbital gyri | Emotions, pain, olfaction, intelligence |
| 12 | Superior to 11, below 10; inferior frontal lobe, prefrontal | As above |
| 13 | Lost in adolescence | |
| 14 | Lost in adolescence | |
| 15 | Lost in adolescence | |
| 16 | Lost in adolescence | |
| Calcarine fissure region, posterior pole, medially | Primary visual cortex, chromatic, luminance | |
| Anterolateral to 17; both superior and inferior, lingual gyrus regions, lateral occipital gyrus | Visual association area, faces | |
| Anterolateral to 18, both superior and inferior, cuneus, lingual, superior occipital gyrus | Visual fields, color, motion | |
| 20 | Inferior temporal gyrus | Visual association |
| Middle temporal gyrus, lateral surface only | Higher order audition, visual association | |
| Posterior-superior temporal gyrus, lateral aspect | Speech reception, auditory association | |
| 23 | Posterior cingulate, medial surface | Emotions, facial familiarity |
| 24 | Anterior cingulate | Emotions, pain, itch, bimanual coordination |
| 25 | Lamina terminalis region, medial perforating substance | ? Olfaction |
| 26 | Posterior commissure region | |
| 27 | Superomedial temporal lobe | |
| Medial surface, anterior-inferior temporal lobe; hippocampus; entorhinal cortex | Gender classification, memories, emotions, olfaction, limbic | |
| 29 | Posterior cingulate, region, posterior induseum griseum | |
| 30 | As above | Visual attention |
| 31 | Precuneus, posterior cingulate | Emotions, pitch of music, familiar faces |
| 32 | Anterior to cingulate, callosomarginal region, anterior internal frontal | Pain |
| 33 | Induseum griseum, anterior cingulate | Emotions |
| 34 | Medial temporal lobe, amygdala, entorhinal | Olfaction, limbic; sadness on left, happiness on right |
| 35 | Inferior temporal lobe, medial aspect; parahippocampus; perirhinal | Limbic, olfaction |
| 36 | Posteroinferior temporal lobe, medial aspect; parahippocampus; fusiform gyrus | Gender classification, memories, emotions, face, information for memories |
| Posterior to 36; extends from medial to lateral inferior temporal lobe, fusiform gyrus | Visual motion, speech, visual association, spatial recognition, naming objects | |
| 38 | Anteromedial tip of temporal lobe, temporopolar | Emotions, pain |
| Angular gyrus of inferior parietal lobe | Face recognition, spatial attention, visual association, making analogies | |
| 40 | Supramarginal gyrus in inferior parietal lobule | Sensory analysis, pain |
| Superior-most temporal gyrus, lateral aspect, anterior transverse gyrus | Primary auditory | |
| Just inferior to 41; superior temporal gyrus | Auditory association, speech recognition | |
| 43 | Frontal opercular region, postcentral gyrus | Language perception |
| Inferior frontal gyrus, opercular region, lateral aspect | Broca’s speech expression, motor toe and finger | |
| Anterior to 44; inferior frontal, lateral aspect, “triangular” gyrus | Broca’s motor speech (posteriorly), tongue movement, upper extremity motor, some perception of speech seen anteriorly | |
| 46 | Anterior to 45; inferior frontal-dorsolateral prefrontal cortex, middle frontal gyrus | Emotions, memory, visual cues |
| 47 | Lateral orbitofrontal | Emotions, familiarity, memory, olfaction, verb generation |
With permission from Mark Dubin, Boulder, Colorado.
Motor System
“I’ve got to scratch that itch!” What brain connections will this simple task require?
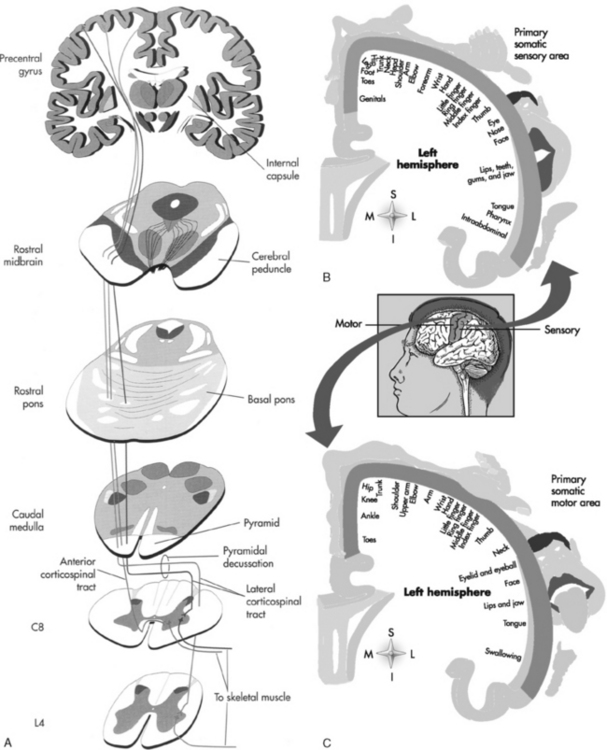
The primary origin of the stimulus for motor function is the precentral gyrus of the frontal lobe, which receives input from many sensory areas (Fig. 2-12; Table 2-3). Stimulation of the motor area of one precentral gyrus causes contraction of muscles on the opposite side of the body. The motor cortex, like the sensory area, is arranged such that the lower extremity is located superomedially along the paracentral lobule in the midline, whereas the upper extremity is located inferolaterally. The cells innervating the hip are at the top of the precentral sulcus; the leg is draped over medially along the interhemispheric fissure. The face (especially the tongue and mouth) has an inordinately large area of motor and sensory representation along the inferiormost aspect of the precentral motor strip on the surface of the brain, just above the sylvian fissure. This picture of the homunculus, with leg hanging over the vertex and with an enormous mouth and tongue, reminds a Baby Boomer of Mick Jagger (Chris Rock for Generation X), with mouth open, jabbering away. The motor contribution to speech is located at the inferior frontal gyrus (frontal operculum regions).
| Pathway | Course | Function |
|---|---|---|
| Lateral corticospinal tract | Primary motor cortex to corona radiata to posterior limb of internal capsule to cerebral peduncle to central pontine region to medulla through pyramidal decussation to posterolateral white matter of cord | Motor to contralateral extremities |
| Anterior corticospinal tract | Primary motor cortex to corona radiata to posterior limb of internal capsule to cerebral peduncle to central pontine region to medulla to anterior funiculus and anterior column of spinal cord | Motor to ipsilateral muscles |
| Rubrospinal tract | Red nucleus to decussation in ventral tegmentum of the midbrain through the lateral funiculus of the spinal cord to the posterolateral white matter of cord (with lateral corticospinal tract) | Motor control of contralateral limbs |
| Reticulospinal tract | Pons and medulla to ipsilateral anterior column of cord | Automatic movement of axial and limb muscles (walking, stretching, orienting behaviors) |
| Vestibulospinal tract | Vestibular nuclei to ipsilateral anterior columns in cord | Balance, postural adjustments, and head and neck coordination |
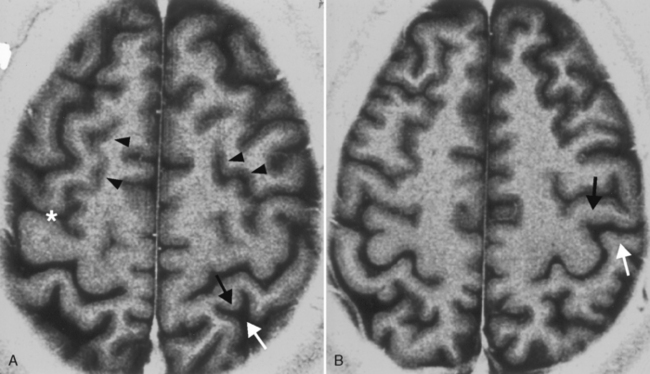
Sometimes finding the central sulcus can be a bear (Fig. 2-13). This is necessary for discriminating motor from sensory areas, particularly when surgery to resect a perirolandic tumor is contemplated. Retaining motor function is desired. Consult Box 2-1 for some clues on how to identify the central sulcus.
Box 2-1 Localization of the Central Sulcus (see Fig. 2-13)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree