Cranial Epidural Abscess and Subdural Empyema
Epidural abscess and subdural empyema are central nervous system infections that arise as infrequent complications of neurosurgical procedures, open trauma, and common infectious processes, such as sinusitis or meningitis. Until penicillin became widely available in the 1940s, mortality from these infections approached 100%. Contemporary reports now cite mortality rates for subdural empyema in the neighborhood of 10%, and mortality from epidural abscess is exceptionally low.1–6 Despite the efficacy of modern antibiotic therapy, timely neurosurgical interventions continue to play an important role in the microbiological diagnosis and control of intracranial hypertension. The coordinated multidisciplinary management of these very sick children yields gratifying clinical recoveries in the great majority of cases.
Epidural abscess, also known as extradural abscess or extradural empyema, is a collection of pus between the dura mater and overlying bone. Subdural empyema is suppuration between the cranial dura and arachnoid membranes. Alternative terms include subdural abscess, purulent pachymeningitis, intradural abscess, and intrameningeal abscess.7,8 Collectively, epidural abscess and subdural empyema are described in this chapter as extracerebral suppuration. The term intracranial suppuration may be used to encompass brain abscess as well as epidural abscess and subdural empyema. The term empyema connotes pus within a preexisting anatomical space, whereas the term abscess describes a collection of pus in the parenchyma of a tissue or an organ. Empyema might therefore be an appropriate term for epidural infection as well, but this chapter conforms to the customary usage, which emphasizes the common focality of epidural pus versus the typically diffuse distribution of subdural pus. This distinction also serves to emphasize the relative benignity of infection limited to the epidural space, as will be seen.6,7,9
78.1 Epidemiology
Little is known about the incidence of extracerebral suppuration in childhood. A population-based study drawing on administrative data sets in the United States estimated the annual incidence of hospital admission for intracranial suppuration, including brain abscess associated with sinusitis or otitis, to be between 3 and 4 per million children per year, but this study did not encompass posttraumatic or postsurgical cases or cases of subdural empyema complicating infantile meningitis.6 The largest case series of extracerebral suppuration have been reported out of Africa and southern Asia,1,2,10–23 but whether these impressive clinical experiences reflect a greater burden of disease or referral to a more limited number of neurosurgical centers from wider geographic regions is impossible to determine. The relative frequencies of epidural abscess and subdural empyema have not been studied in epidemiologic terms, but case series that include both entities tend to cite comparable numbers of each.3,8,9,11,24–28 Exceptions are several large case series from Africa that show a marked predominance of subdural empyema.2,13,22 Mixed epidural and subdural infections and associated brain abscesses are not uncommon.3,11,27,29–31 Empyema associated with infantile meningitis is exclusively subdural. The etiology of the infection determines the typical age at presentation. Subdural empyema complicating meningitis is a phenomenon of infancy and early childhood.8 The incidence of intracranial suppuration complicating sinus and ear disease peaks in mid adolescence.2,6,11,13,22,32–34 Regardless of the etiology, a male predominance has been observed consistently at a rate of roughly 2:1.
The dominant etiology of epidural abscess and subdural empyema in childhood is extension from head and neck infection. The facial sinuses, middle ear, and mastoid sinus are the most common primary sites. Subdural empyema complicating dental infection has been reported, but in distinction to its prominent role in the etiology of brain abscess, it accounts for a negligible fraction of cases of extracerebral intracranial suppuration.2,35,36 In infancy and early childhood, subdural empyema is seen most commonly as a complication of meningitis.8,11,32,37 The following discussion proceeds along these etiologic lines. Posttraumatic and postsurgical infections are fortunately infrequent and are necessarily contingent on preceding trauma or surgery. They are considered only very briefly.
78.2 Sinusitis and Ear Disease
78.2.1 Incidence
Sinusitis and ear disease are very common in childhood, but the incidence of these conditions is poorly defined. The clinical diagnosis is imprecise, and many cases escape medical attention altogether.5,28,38 Outpatient management is the rule, and only a small but unknown fraction of patients are admitted to a hospital for parenteral antibiotics. Among 649 patients of all ages hospitalized for sinusitis, Clayman et al recorded intracranial complications, including brain abscess and meningitis as well as extracerebral abscess, in 24 (3.7%).38 Analysis of the 2009 Kids’ Inpatient Database (KID) disclosed an estimated 10,906 nationwide admissions with a primary diagnosis of sinusitis, otitis, or mastoiditis. That year, there were an estimated 443 admissions for intracranial suppuration of all types associated with sinusitis or ear disease, for a rate of 4.1% (J.H.P., unpublished data). Thus, the incidence of extracerebral suppuration complicating sinusitis or ear disease can be taken to be less than 1/25th the incidence of hospitalization for these conditions and to be a very tiny fraction of the overall incidence. The miniscule incidence of intracranial suppurative complications of sinusitis has frustrated efforts to prevent them.
78.2.2 Mechanism
Extracerebral suppuration is usually associated with sinusitis involving the frontal or ethmoid sinuses.7,39 The next most commonly involved sinus is the mastoid.40
Speculation about the mechanisms of spread of infection from the sinuses to the epidural and subdural spaces has been based on gross observations at surgery and at autopsy. Seldom are macroscopic defects observed in the posterior wall of the frontal sinus or the roof of the ethmoid sinus, but in the setting of chronic otitis and mastoiditis, destruction of the roof of the middle ear, the tegmen tympani, can be seen in association with epidural pus on the floor of the middle fossa (▶ Fig. 78.1a,b). The perforations of the cribriform plate have been mentioned as possible portals for entry of infection, but they are likely more relevant to the pathogenesis of meningitis than of extracerebral suppuration. In the absence of an osseous defect, infection is commonly believed to spread by septic thrombophlebitis of the transosseous venous channels. Spread through the dura may occur on a similar basis. At autopsy, frank necrosis of the dura has been observed adjacent to an infected sinus, accounting for some instances of the breach of what is usually a very stout barrier to the spread of infection.7 Septic thrombosis of cortical bridging veins and adjacent dural venous sinuses is more likely a complication of advanced subdural empyema than a mechanistic cause.
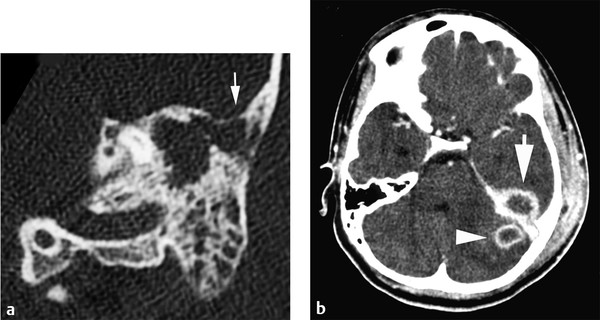
Fig. 78.1 (a) Computed tomographic (CT) scan of the temporal bone of a child with otitis and mastoiditis shows opacification of the antrum and mastoid cells with erosion of the tegmen tympani (white arrow). (b) CT scan of the brain with enhancement in the same patient demonstrates a small abscess in the left cerebellar hemisphere (white arrowhead) and an extracerebral abscess on the floor of the middle fossa (white arrow). At surgery, the middle fossa collection proved to be epidural.
(Courtesy of Drs. Joseph Piatt, Jennifer Smith, and Eric Faerber.)
78.2.3 Predisposing Factors
Male sex, black race, and age in the early teens seem to impart a vulnerability to intracranial suppurative complications of sinusitis and ear disease. The developmental, anatomical, and physiologic mechanisms underlying these associations have been the subject of speculation, but they are unknown.6,26 Factors that contribute to the development of sinusitis necessarily make indirect contributions to the incidence of its complications. Many studies have described a seasonal pattern that seems dependent on geography. In central Europe, cases of complicated sinusitis peak in the month of March,40 and reports from Africa and India find most cases of subdural empyema in their summer months.4,10,12 In the United States, winter is the dominant season for sinogenic intracranial suppuration,30 but no seasonal pattern is apparent for otogenic cases.6 In view of the anatomical, physiologic, and immunologic continuity of the sinobronchial tree, there has been much discussion of the association of sinusitis with allergy and asthma. A large cohort study in the United States identified a strong association of asthma with sinogenic intracranial suppuration. This association did not hold for ear disease.6 The linkage between sinogenic intracranial suppuration and asthma raises questions about the relevance of environmental factors like air quality.41 Over the first decade of this century, there has been no clear trend in the incidence of sinogenic intracranial suppuration.6 Analysis of the 2009 KID shows that patients admitted with a primary diagnosis of sinusitis, otitis, or mastoiditis came from ZIP codes where the median home income was lower than those for other admissions, but this association was not evident for intracranial suppuration complicating sinusitis and ear disease (J. H. P., unpublished data). Adame and colleagues have discussed a possible linkage of sinogenic empyema with diabetes, another prevalent and chronic condition with social and environmental associations.42 Geographic and socioeconomic correlations deserve further study.
78.2.4 Clinical Presentation
The initial clinical presentation of epidural abscess complicating sinusitis or ear disease is indistinguishable from the symptoms and signs caused by the primary infection: headache, fever, sinus pain or tenderness, purulent rhinorrhea or otorrhea, and conductive hearing loss. Only later in the course of the illness, if the epidural collection attains sufficient volume to cause mass effect or if complicating subdural suppuration or cerebral abscess develops, can papilledema, focal neurologic deficits, depressed responsiveness, and seizures appear. A special case is the distinctive clinical syndrome of Pott’s puffy tumor (▶ Fig. 78.2). Named after Sir Percivall Pott, an English surgeon who described this phenomenon in 1760, the puffiness reflects subperiosteal pus and osteomyelitis of the frontal bone complicating frontal sinusitis. It is reliably associated with at least some degree of epidural suppuration, and in more advanced cases, subdural empyema or cerebral abscess may be present as well. Pott’s puffy tumor has been described as a historical curiosity,43 but it is certainly a feature of contemporary practice.11,25,27,42,44–48 Osteomyelitis of the frontal bone raises particular issues for treatment that are discussed below.
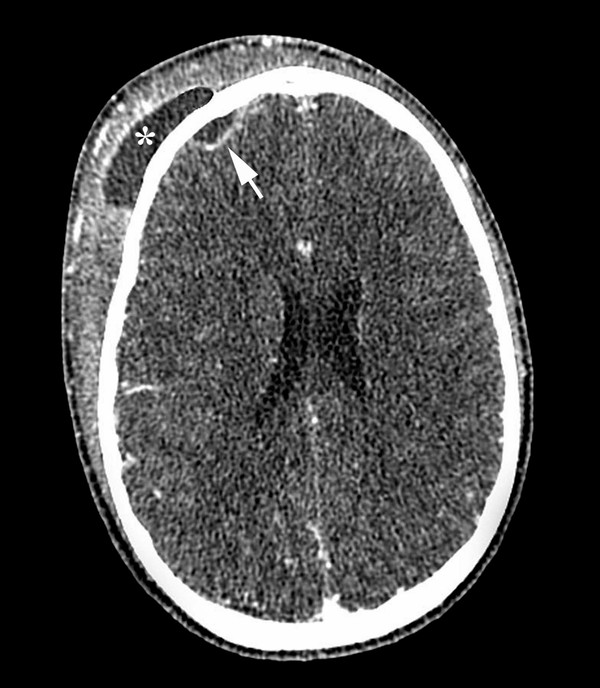
Fig. 78.2 Computed tomographic scan with enhancement of the brain in a case of frontal sinusitis with Pott puffy tumor. The scan shows a large subperiosteal abscess (asterisk) and a smaller epidural abscess (arrow).
(Courtesy of Drs. Joseph Piatt, Jennifer Smith, and Eric Faerber.)
Subdural empyema complicating sinusitis or ear disease may be impossible to distinguish from meningitis in its initial clinical manifestations; fever, headache, lethargy, and meningismus are common. The development of focal neurologic deficits and seizures directs attention to the correct diagnosis. Before the current era of computed brain imaging, the clinical triad of fever, seizures, and hemiplegia was viewed as pathognomonic for subdural empyema.
78.2.5 Microbiology
Streptococcus and Staphylococcus species are the most common isolates.4,5,9–11,13,27–31,33,49–53 Most frequently mentioned by genus and species is Streptococcus milleri. In contemporary parlance, this term refers to a group of α-hemolytic viridans streptococci—Streptococcus anginosus, Streptococcus constellatus, and Streptococcus intermedius, the so-called Streptococcus milleri group (SMG).54 SMG organisms are typically pyogenic and tend to be found in association with the gastrointestinal tract, oropharynx, facial sinuses, and teeth. Gram-negative organisms, particularly Pseudomonas, are prevalent in cases of otogenic suppuration associated with cholesteatoma.55,56 The meticulous handling of specimens discloses substantial rates of anaerobic and polymicrobial infection that must be considered in treatment planning.29,53,57–59 In geographic regions where it is prevalent, tuberculosis is a possibility as well.60 Intracranial pus yields positive cultures in upward of 65% of cases.2,4,5,10,11,13,16,25,27,29,31,33,34,44,50–53,57,61
Once the existence of sinogenic or otogenic intracranial suppuration has been recognized, lumbar puncture is no longer indicated. As a diagnostic priority, lumbar puncture is superseded by the sampling of pus from the intracranial collections and the sinuses, and in the presence of intracranial mass effect, lumbar puncture may be contraindicated because of the risk for herniation, a complication mentioned frequently in the older literature.13,62,63 Culture-positive cerebrospinal fluid (CSF) has been reported in fewer than half of cases of subdural empyema, and in many reports the culture yield is nil.5,13,20,42,51,52,62,64–66 The CSF formula generally conforms to a parameningeal pattern: a neutrophil-predominant pleocytosis with elevated protein and normal glucose.62 The CSF findings associated with epidural abscess have received little attention in the literature.2
Blood cultures are obtained routinely and have been reported to be positive in up to 40% of cases.5,10,27,50,52 Other laboratory studies, such as the white blood cell count, erythrocyte sedimentation rate, and C-reactive protein level, are unlikely to impact the diagnostic process, but they may be useful to follow the response to treatment. Reports from two centers have noted that although the C-reactive protein level and erythrocyte sedimentation rate are consistently elevated in patients with sinusitis, these markers are much higher in cases complicated by intracranial suppuration.27,42
78.2.6 Imaging
The decision for brain imaging in the patient with suspected intracranial suppuration seldom lies in the hands of the neurosurgeon, but for monitoring the patient’s response to treatment, computed tomography (CT) and magnetic resonance (MR) imaging both have strengths and weaknesses that must be considered.
CT is adequate for most neurosurgical purposes. Extracerebral suppuration generally exhibits low density compared with brain (▶ Fig. 78.3a). Epidural abscess has the expected biconcave morphology (▶ Fig. 78.5), and subdural empyema spreads more widely and conforms more closely to the contour of the adjacent brain. With the administration of contrast, there is pachy- and leptomeningeal enhancement surrounding the collection (▶ Fig. 78.3b). An advantage of CT is precise demonstration of the osseous anatomy and pathology in the calvaria and in the facial sinuses and temporal bones. The prevailing navigation systems for endoscopic sinus surgery, often undertaken by colleagues in otolaryngology concurrently with the neurosurgical drainage of intracranial pus, rely on CT data sets. A disadvantage of CT is the artifactual image distortion that occurs at the interface between high-density bone and adjacent low-density soft tissue, so-called beam hardening. Unfortunately, in this clinical context the regions of the cranial cavity immediately adjacent to the skull are of paramount interest—the epidural and subdural spaces and the dural venous sinuses. Another potential weakness of axial CT relates to the evaluation of lesions at the vertex of the cranial cavity, a not uncommon site for epidural abscess.8 Sagittal and coronal reconstructions obviate this problem. Without a doubt, CT has poorer image clarity and lower sensitivity than MR imaging. When CT is employed as an initial diagnostic test, the occurrence of false-negative results of noncontrast CT must be born in mind,27,42,67–69 and if intracranial infection is suspected prospectively, contrast must be administered.70
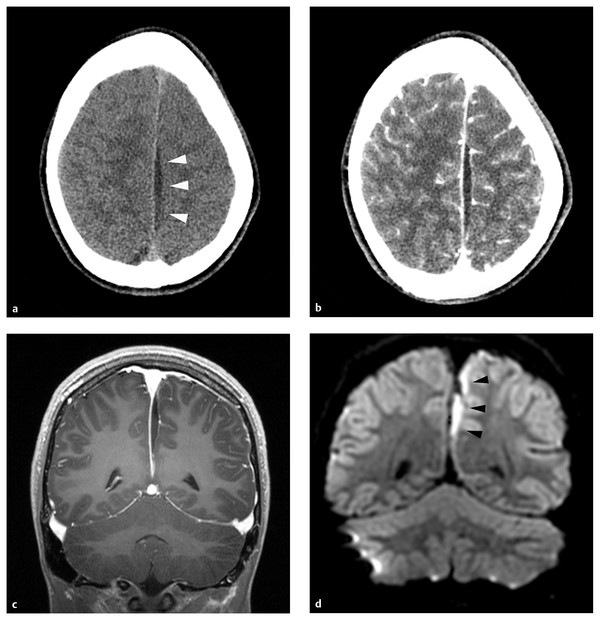
Fig. 78.3 (a) This school-age boy had headache and fever. He was lethargic at presentation, and there was a right lower limb monoparesis. After functional endoscopic sinus surgery for his associated frontal and ethmoid sinusitis, he received a 6-week course of parenteral antibiotics. There was no neurosurgical intervention. He recovered completely. Computed tomographic scan of the brain without contrast shows a small, low-density collection in the interhemispheric fissure (white arrowheads). (b) There is pachy- and leptomeningeal enhancement after contrast administration. (c) Likewise, there is pachy- and leptomeningeal enhancement on this coronal, T1-weighted magnetic resonance imaging sequence. The collection itself is low-intensity compared with brain. (d) On diffusion-weighted imaging, the interhemispheric pus is high-intensity (black arrowheads).
MR imaging offers much more anatomical and physiologic information than CT, but whether this information justifies the additional logistical complexity and commitment of time requires consideration on a case-by-case basis. Epidural and subdural pus are generally low-intensity on T1-weighted and fluid-attenuated inversion recovery (FLAIR) sequences and high- or mixed-intensity on T2-weighted sequences.71 Enhancement patterns are similar to those on CT (▶ Fig. 78.3c). Diffusion-weighted imaging (DWI) detects ischemic injury in brain adjacent to subdural collections, and because the diffusion of water protons is restricted by the viscosity of pus, the collections themselves are distinct high-signal structures (▶ Fig. 78.3d)71–73 Conversely, purulent collections have low intensity on apparent diffusion coefficient (ADC) maps.71,72 Epidural abscesses may not restrict diffusion so consistently as subdural collections.74 MR spectroscopy detects the elevated lactate concentrations typical of purulent collections. Arterial spin labeling sequences document cerebral perfusion, and MR venography offers sensitive assessment of the dural venous sinuses. Many of these data, elegant as they are by historical standards, are of limited practical usefulness for treatment planning. The utility of imaging data for the prognostication of functional outcomes has not been analyzed extensively, but patients with cortical ischemic changes on DWI sequences in the acute stages of their illness often exhibit encephalomalacic changes at the corresponding sites on late follow-up (▶ Fig. 78.4a–c). MR imaging is certainly the most sensitive technique for identifying small purulent collections adjacent to the skull and thus may be the technique of choice for monitoring response to antibiotic treatment.
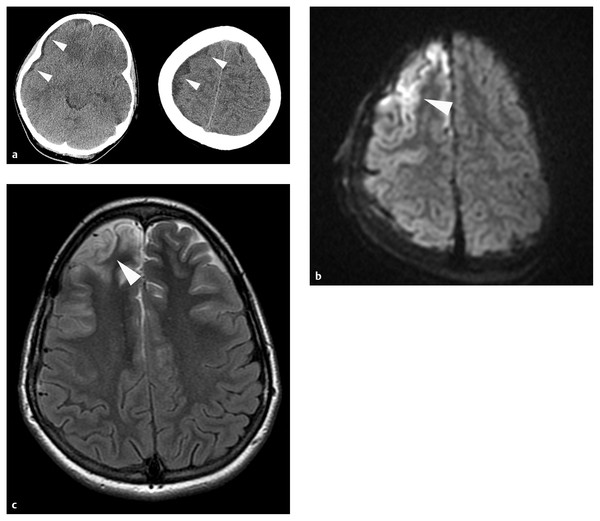
Fig. 78.4 (a) This school-age girl collapsed at home after several days of headaches and fevers. In the emergency department, she was noted to be hemiplegic on the left side. A computed tomographic scan of the head without contrast shows a thin, low-density subdural collection (white arrowheads) distributed widely over the right convexity. She underwent immediate osteoplastic right frontoparietotemporal craniotomy for drainage of her empyema. Her postoperative course was notable for septic shock requiring aggressive fluid resuscitation and pressors. (b) The diffusion-weighted image (DWI) from a study performed on the third hospital day shows high signal intensity in the cortical mantle of the right frontal lobe (white arrowhead). (c) At late follow-up, her hemiplegia had resolved completely, but she has persisting neurocognitive disabilities and is continuing in rehabilitation. This T2-weighted axial MR imaging sequence shows encephalomalacia (white arrowhead) at the site of the abnormality on the earlier DWI sequence.
78.2.7 Treatment
The primary treatment of intracranial suppuration of sinogenic or otogenic origin is antimicrobial.11 The presence of subdural or epidural pus is not by itself an indication for surgical drainage. Complete drainage of intracranial pus is not possible and is not necessary for cure. Indeed, in a recent cohort study based on a large administrative data set, more than 25% of patients with intracranial suppuration of sinogenic or otogenic origin were managed nonoperatively.6 The indications for surgical intervention are twofold: to obtain a microbiological diagnosis and to relieve symptomatic or threatening mass effect. The former indication often motivates urgent neurosurgical intervention at the time of presentation. The latter indication is clearly a matter for judgment based on careful assessment of the patient’s clinical condition and imaging data. It may be present at presentation, or it may arise at unpredictable times during the initial phase of treatment.53 Surveillance imaging at frequent intervals allows close monitoring of the size and distribution of subdural collections.
A detailed discussion of antibiotic selection is beyond the scope of this chapter. In principle, therapy is guided by the sensitivities of the microbial isolates, but actual practice is not so intellectually satisfying. The administration of antibiotics before specimens can be obtained may compromise the sensitivity of culture methods. Although the concordance between intracranial cultures and sinus or ear cultures has been reported to be good,10,53,75 the relevance of extracranial cultures to intracranial suppuration is always uncertain, and the isolation of one organism does not eliminate the possibility of polymicrobial infection. Typically, three parenteral antibiotics must be employed at the outset to cover staphylococci, streptococci, gram-negative organisms, and anaerobes, and regardless of culture results, most patients are subjected to 6 weeks of broad-spectrum therapy.
Questions of surgical technique have been the topic of animated discussion in the literature, particularly for subdural empyema. Because surgery commonly must be undertaken on an urgent basis at odd hours, circumstances may favor the speed and simplicity of bur holes, and surgeons who feel compelled to discard craniotomy flaps elevated in the presence of pus naturally reserve craniotomy for patients who fail to respond to more limited interventions.51 This concern is misplaced. Craniotomy flaps elevated with an osteoplastic technique are highly resistant to infection, and under contemporary broad-spectrum antibiotic coverage, even free flaps heal reliably without complications.76 On the other hand, many authorities have commented that patients treated initially with bur holes are subject to more frequent returns to the operating room for recurrent or new purulent collections than patients treated initially by craniotomy.1,8,13,16,77,78 In the very large case series of Nathoo and associates, craniotomy was associated with improved outcomes as well.1 Craniotomy at presentation is the senior author’s usual practice—an osteoplastic frontoparietotemporal craniotomy large enough for wide exposure of the more involved convexity and cautious drainage of the interhemispheric fissure, if necessary. And whatever may be the first intervention, reoperation for the reaccumulation of pus is so frequently necessary that this contingency must be born in mind in planning the initial exposure. Reoperation rates between 9 and 50% have been reported.1,5,8,77
Subdural empyema of the posterior fossa is often diffusely distributed over the tentorial, petrosal, and occipital surfaces of the cerebellum, so a wide exposure is required.12 Transient swelling of the cerebellum may necessitate leaving the craniotomy flap out and the dura unclosed. The senior author’s usual practice in midline exposure of the posterior fossa is to leave a myofascial cuff along the nuchal line to permit reattachment of the cervical muscles to the occipital bone in the closure. This maneuver minimizes deformity and may reduce the risk for CSF–wound fistula when the dura must be left open in a large craniectomy defect. Posterior fossa subdural empyema is often complicated by hydrocephalus, which may be transient or persistent.10 In the large case series of Nathoo and associates, hydrocephalus developed in 77% of cases.12 External ventricular drainage is required in the acute phase, and a CSF shunt may be considered after the infection has been eradicated.
Surgery for epidural abscess differs in some respects. If a microbiological diagnosis has been obtained from sinus cultures, small collections may be managed nonoperatively.46 Epidural abscesses are generally more focal than subdural empyemas, so drainage by bur holes often suffices.2 Because most epidural abscesses are frontal, cosmetic considerations are pertinent. A bicoronal scalp incision for the drainage of frontal epidural collections can sometimes be avoided by employing an infraciliary incision and a small bur hole in the orbital roof to gain access to the anterior fossa directly or through the frontal sinus (▶ Fig. 78.5). The special case of Pott’s puffy tumor raises the question of how to manage the associated osteomyelitis of the frontal bone. In the absence of a frank sequestrum, surgical restraint is in order. Craniectomy of the involved bone creates a skull defect with appalling cosmetic consequences and is generally unnecessary. Even a badly eroded frontal bone has the potential to heal solidly with a long course of antibiotic therapy (▶ Fig. 78.6a–d).
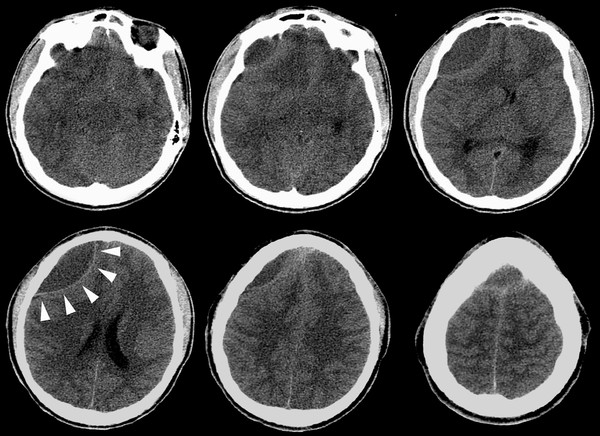
Fig. 78.5 This computed tomographic scan of the brain without enhancement shows a large anterior fossa epidural abscess. White arrowheads mark the biconcave contour. This collection was drained successfully through an infraciliary incision and a bur hole in the right orbital roof.









