8 Cranial Nerve VIII
Auditory and Vestibular
Auditory Nerve
Anatomy
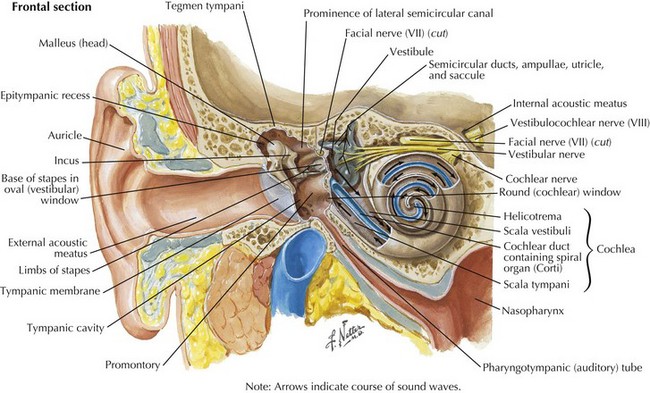
Sound waves travel through the external auditory canal and vibrate the TM, which in turn produces motion of the middle ear ossicles (incus, malleus, and stapes). The vibrations are transmitted through the oval window at the footplate of the stapes, causing a wave to travel through the endolymphatic fluid of the cochlea of the inner ear. The fluid waves vibrate the organ of Corti’s basilar membrane, stimulating inner and outer hair cells (Fig. 8-1). Hair cells, receptors of the sensorineural system, transmit action potentials to bipolar neurons, the bodies of which are in the spiral ganglion.
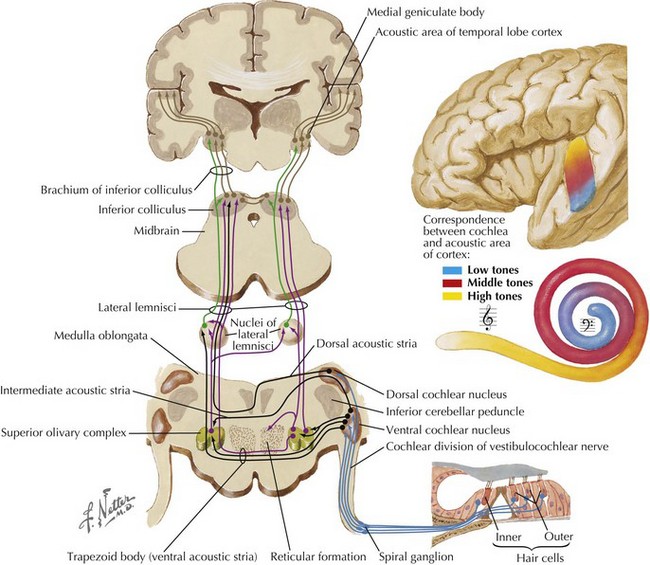
Afferent fibers projecting toward the CNS comprise the auditory nerve (Fig. 8-2). They travel to the dorsal and ventral cochlear nuclei located in the caudolateral pons. Most of the secondary neurons project contralaterally across the midline to the superior olivary nucleus and then travel up the lateral lemniscus into the inferior colliculus of the midbrain. Decussating fibers from the cochlear nucleus to the superior olivary nucleus are located in the trapezoid bodies and also in the base of the pons. Fibers from the inferior colliculus continue to travel rostrally to the medial geniculate body of the thalamus and then terminate in the auditory cortex located in the transverse temporal gyri of Heschl.
Clinical Presentation
Physical Examination
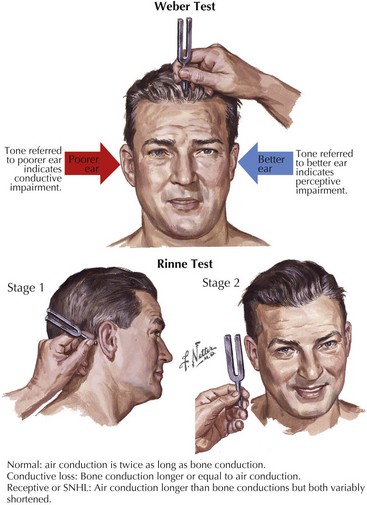
Tuning fork tests assess whether the hearing loss is conductive or sensorineural (Fig. 8-3). During the head and neck examination, a complete cranial nerve examination must also be performed to assess other potential cranial nerve abnormalities. Facial nerve weakness may be attributed to viral infections, such as herpes zoster oticus, or expanding neoplasms in the internal auditory canal or cerebello-pontine angle, such as meningiomas or facial neuromas. Auscultation of the areas around the orbit and ear may detect pulsatile tinnitus. The type of SNHL can assist in the localization of the lesion. Ototoxic drugs, excess noise exposure, and autoimmune diseases affect the hair cells within the cochlea, the primary sensory organ of hearing, and lead to hearing loss usually described as decreased sensitivity to pure tones but preserved speech discrimination. Hearing loss caused by retrocochlear lesions of the nerve fibers of CN-VIII or its central auditory projections begins as decreased speech discrimination with relatively normal pure-tone sensitivity. However, decreased speech discrimination is not exclusive to retrocochlear lesions; it is also observed with extensive hair cell damage.
Differential Diagnosis
Neoplasms
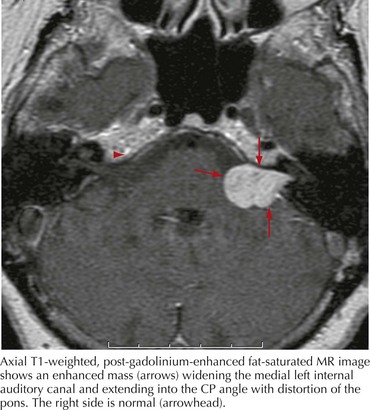
In any case of sudden, unilateral hearing loss, neoplastic lesions, although rare, should be considered in the differential until excluded by diagnostic or radiologic testing. Vestibular schwannomas (also known as acoustic neuromas) are benign tumors arising from the Schwann cells of CN-VIII and account for 6% of all intracranial tumors (Fig. 8-4). These occur on the vestibular portion of CN-VIII and involve the adjacent cochlear division by compression against the bony walls of the internal auditory canal. Less commonly, neuromas can also arise directly from the cochlear nerve.
Vascular Etiologies
Vertebrobasilar stroke is another cause of sudden, unilateral SNHL with potentially devastating effects. Distinguishing whether hearing loss results from microvascular disease or a brainstem infarct is vital. The anterior inferior cerebellar artery supplies blood to the inferolateral portion of the pons, CN-VII, the spinal trigeminal tract, and the inferior cerebellum. A stroke from occlusion of this artery causes an infarct of the ipsilateral pons, creating a myriad of symptoms: ipsilateral hearing loss and vestibular symptoms, gait ataxia, conjugate gaze palsy, ipsilateral facial paralysis and often contralateral loss of pain and temperature sensation in the extremities (see Chapter 55).