9 Cranial Nerves IX and X
Glossopharyngeal and Vagus
Cranial Nerve IX: Glossopharyngeal Nerve and Swallowing
Physiology
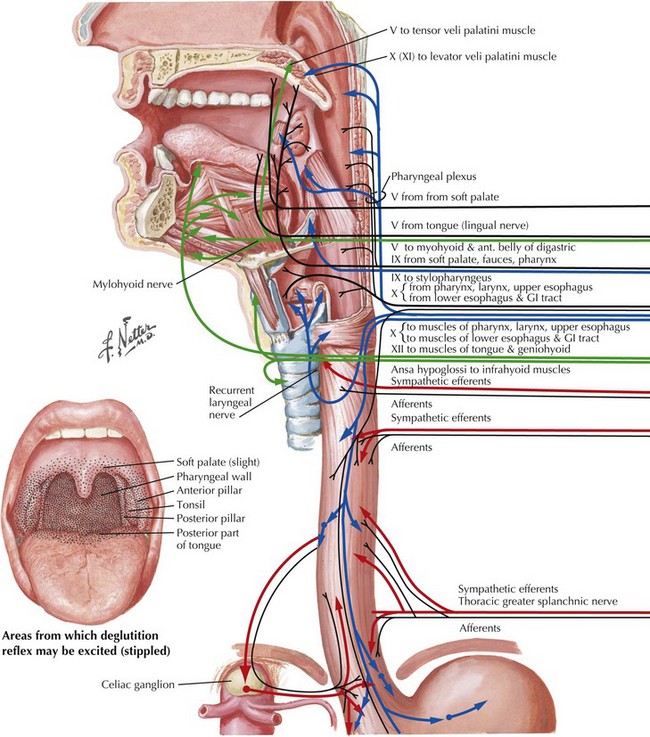
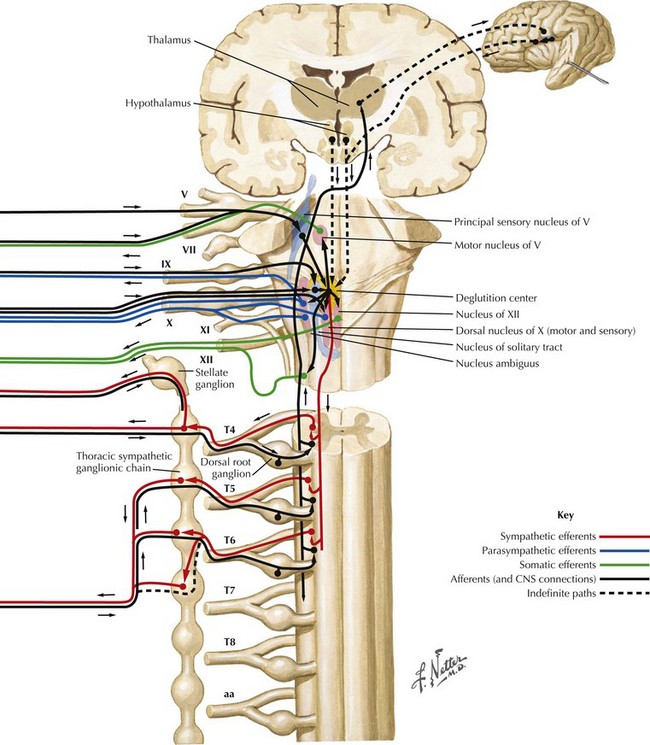
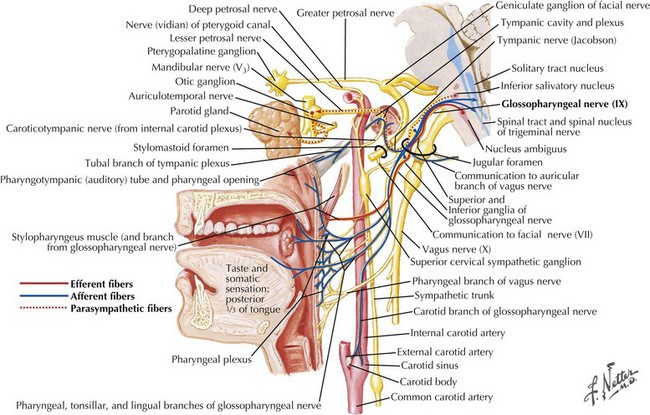
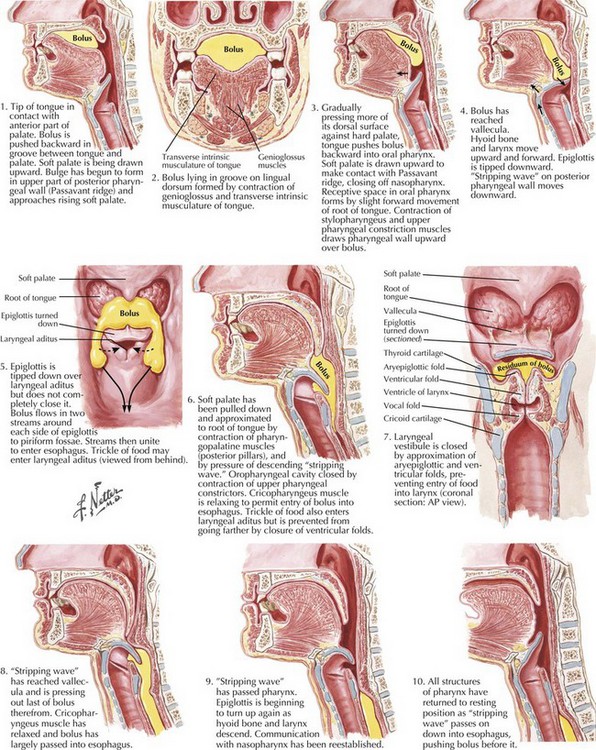
Swallowing is a complex process involving motor control with sensory feedback from many anatomic structures within the oral cavity, pharynx, larynx, and esophagus (Fig. 9-1). The trigeminal (CN-V), facial (CN-VII), glossopharyngeal (CN-IX; Fig. 9-2), vagus (CN-X; Fig. 9-3), and hypoglossal (CN-XII) cranial nerves are involved. A “normal swallow” comprises two major components, bolus transport and airway protection. The swallowing process is typically classified into four phases (Fig. 9-4).
1. The oral preparatory phase involves voluntary motor function during which food or liquid is taken into the mouth, masticated (CN-V), and mixed with saliva to form a cohesive bolus (Fig. 9-4, nos. 1 and 2). This phase requires coordination of several cranial nerves and corresponding structures. Tension in the labial and buccal musculature closes the anterior and lateral sulci (CN-VII) while rotary mandible motion produces chewing (CN-V3). Lateral rolling tongue motion (CN-XII) and bulging of the soft palate forward (widening the nasal airway, and sealing the posterior oral cavity) properly positions the bolus for the swallowing (CN-IX). Tongue mobility is the most important neuromuscular function involved in this first phase. The mid and lower divisions of CN-V provide sensory feedback for positioning the bolus. Saliva derived from the parotid, sublingual, and submandibular glands (innervated by secretomotor fibers of CN-IX and -VII) contain digestive enzymes that act as an emollient to soften and shape the bolus.
2. The oral swallowing phase is initiated when the tongue (CN-XII) sequentially squeezes the bolus posteriorly against the hard palate and initiates propulsion into the oropharynx (Fig. 9-4, nos. 3 and 4). Lips and buccal muscles contract (CN-V and CN-VII) with elevation of the velum (CN-V and CN-X) providing the valving process that generates pressure to seal the nasopharynx, preventing reflux and nasal regurgitation. CN-V is responsible for the afferent (sensory) feedback for the entire oral cavity and tongue. The soft palate (CN-IX), critical to containing the bolus within the oral cavity during the oral preparatory phase, now moves posteriorly to allow the bolus to pass through the faucial arches and simultaneously prevent the bolus from entering the nasopharynx. The swallowing reflex is triggered as the bolus passes the anterior tonsillar pillars, which initiates the pharyngeal phase.
3. The pharyngeal phase begins with the bolus passing into the throat, triggering the swallowing reflex and causing several pharyngeal physiologic actions to occur simultaneously, allowing food to pass into the esophagus (Fig. 9-4, nos. 5–7). Once pharyngeal swallowing is elicited, essential functions of airway protection occur. Intrinsic laryngeal muscles innervated by CN-X close the larynx at the aryepiglottic, false vocal, and at the true vocal folds, creating a seal that separates the airway from the digestive tract protecting the laryngeal vestibule from foreign material aspiration. The tongue (CN XII) is the major force pushing the bolus through the pharynx. Synergistic actions with CN-X produce pharyngeal peristalsis as it innervates the pharyngeal constrictors and carries afferents from the lower pharynx.
Poor airway protection and delayed triggering of pharyngeal swallow may cause aspiration. When swallowing is inefficient and aspiration occurs, a reflexive cough needs to occur as a respiratory defense against foreign matter. The cough reflex is induced by irritation of afferent CN-IX and CN-X sensory fibers in the larynx, trachea, and larger bronchi (Figs. 9-2 and 9-3). If a reflexive cough is not elicited in response to foreign material within the airway, silent aspiration results; it is radiographically documented in 50% of aspiration cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree