Craniopharyngiomas
Cushing graphically described craniopharyngiomas as “the kaleidoscopic tumors, solid and cystic which take their origin from epithelial rests ascribable to an imperfect closure of the hypophyseal or craniopharyngeal duct” and whose management is “one of the most baffling problems to the neurosurgeon.”1 The benign histology of these tumors is often in marked contrast to their malignant clinical course in children. The location of craniopharyngiomas, which have an intimate association with the visual pathways, hypothalamus, and limbic system, predisposes patients with these tumors to severe visual, endocrine, and cognitive deficits, both at presentation and as a result of treatment. Although most children can compensate for neurologic deficits and endocrinologic deficiencies, the cognitive and psychosocial sequelae may be functionally devastating, interfering with education, limiting independence, and adversely affecting the quality of life as the children approach adulthood.2
37.1 Epidemiology
Craniopharyngiomas constitute approximately 3% of all intracranial neoplasms.3,4 They are the most common nonglial tumor of childhood, accounting for 6 to 9% of pediatric brain tumors.5–7 Although craniopharyngiomas comprise a significant proportion of pediatric brain tumors, on a population basis they are rare. Based on an analysis of three population-based cancer registries, the incidence of craniopharyngioma in the United States is between 0.13 per 100,000 and 0.18 per 100,000 per person-years.8 A bimodal distribution by age has been noted, with peak incidence rates in children and among older adults. Among children, the incidence is greatest between the ages of 6 and 10 years, followed by the ages of 11 and 15 years.8,9 From 33 to 54% of all craniopharyngiomas occur in the pediatric age group,6,8,10–14 with approximately 96 to 145 new cases annually occurring in children from 0 to 14 years of age.
Although there does not appear to be any racial or ethnic predilection for craniopharyngiomas, the influence of gender is unclear. After all the data are considered, craniopharyngioma may occur slightly more often in boys.8,13,15–17
Stiller and Nectoux18 have reported that the proportion of brain tumors that are craniopharyngiomas varied substantially among different global regions: 1.5% in Australia, 4.7 to 7.9% in Europe, 3.9% in Japan, 2.7% (Caucasians) and 4.9% (African Americans) in the United States, and 11.6% in Africa. Although this international variation in occurrence has led to speculation regarding environmental influences, the data must be interpreted with caution because socioeconomic conditions preclude the population-based reporting of all brain tumors in developing countries.
37.2 Pathology
Craniopharyngiomas develop from epithelial nests that are embryonic remnants of the Rathke pouch located on an axis extending from the sella turcica along the pituitary stalk to the hypothalamus and floor of the third ventricle.4,19 Craniopharyngiomas gradually enlarge as partially calcified solid and cystic masses predominantly in the suprasellar region, and the cystic component can reach several centimeters. They extend along the path of least resistance into the basal cisterns or can invaginate the third ventricle. With continued growth superiorly into the third ventricle, hydrocephalus may develop.
Craniopharyngiomas have two basic patterns of cellular growth: adamantinomatous and papillary.3,4,10,14,20,21 Mixed tumors with both adamantinomatous and squamous papillary components or combinations of craniopharyngioma and Rathke cleft cysts can occur.3,4,10,14,20–23
The adamantinomatous tumors are the most common variant, occurring at all ages. They resemble the epithelium of tooth-forming tumors, containing three distinct components: a basal layer of small cells; an intermediate layer of variable thickness with loose, stellate cells; and a top layer facing the cyst lumen, where the cells are abruptly enlarged, flattened, and keratinized. At the cyst surface, desquamated epithelial cells are present either singly or in characteristic stacked clusters (keratin nodules). These nodules may undergo mineralization with the accumulation of calcium salts, which in rare instances progresses to metaplastic bone formation. The cysts in adamantinomatous craniopharyngiomas usually contain an oily liquid composed of this desquamated epithelium, which is rich in cholesterol, keratin, and occasionally calcium.
Squamous papillary craniopharyngiomas occur nearly exclusively in adults and tend to involve the third ventricle.24 They consist of solid epithelium, without loose stellate zones, in a papillary architecture resembling that of metaplastic respiratory epithelium.4,10,21,24 They are predominantly solid and rarely undergo mineralization. When cysts occur, the fluid is less oily and dark than in adamantinomatous tumors. As a result of the absence of calcification and minimal cyst formation, complete curative surgical resection may be obtained more often than with adamantinomatous or mixed craniopharyngiomas.10,17,23 Histology does not affect the risk for recurrence after subtotal resection or the response to radiation therapy (RT).
Microscopic islets or “fingers” of adamantinomatous tumor embedded in densely gliotic parenchyma are frequently seen when the tumor arises in the region of the tuber cinereum, hypothalamus, and floor of the third ventricle.21,23,25–29 The gliotic reaction of Rosenthal fibers and fibrillary astrocytes, varying between several hundred microns to millimeters in thickness,26 effectively separates tumor from brain, thus providing a safe plane for surgical dissection.12,23,28,30 The presence of this gliotic tissue on surgical pathology is associated with a decreased risk for recurrence following a gross total tumor resection.23
37.3 Radiology
The role of neuroimaging is to establish a preoperative diagnosis and then define the location and extent of the cystic, solid, and calcified portions of the tumor and its relationship to the distorted normal anatomy. Radiographic evaluation includes computed tomography (CT), magnetic resonance (MR) imaging, MR angiography, and, where available, MR spectroscopy.31–33 Vascular anatomy can be well demonstrated by MR imaging and MR angiography, obviating the need for invasive cerebral angiography.32
CT and MR imaging have complementary roles in the diagnosis of craniopharyngiomas31,32,34 (▶ Fig. 37.1). CT is superior in the detection of the varied and complex calcifications. Noncontrast CT usually demonstrates a suprasellar and often intrasellar mass with calcifications, as well as hypodense solid and cyst components. The low density of the cystic component is usually greater than the attenuation of cerebrospinal fluid (CSF). A small percentage of craniopharyngioma cysts may be of high density.31 CT shows secondary changes in the skull base, such as enlargement of the sella turcica and/or erosion of the dorsum sellae. When MR imaging is available, contrast-enhanced CT is unnecessary.
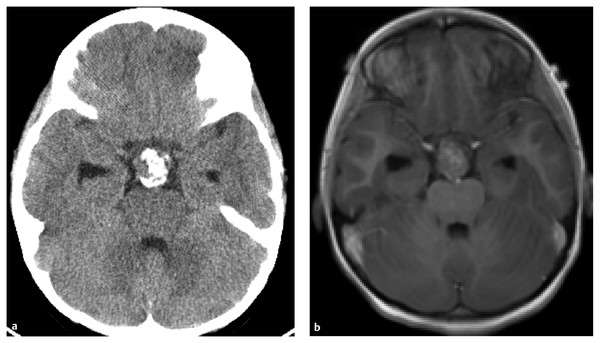
Fig. 37.1 (a) Noncontast computed tomographic (CT) scan demonstrating calcified portion of tumor. (b) Corresponding contrast-enhanced T1 axial magnetic resonance image. Note the nonenhancing portion, representing the calcification seen on CT.
MR imaging and MR angiography provide valuable information about the relationships of the tumor to surrounding structures, delineating the involvement or displacement of the visual pathways, hypothalamus, ventricles, and vessels of the circle of Willis. Noncontrast sagittal T1-weighted images may show the normal pituitary, leading to the correct diagnosis.34 Fine calcifications may not be visible, demonstrating a paradoxically increased signal on T1 imaging or, if more substantial, exhibiting characteristic signal voids. Craniopharyngioma cysts are uniformly bright on T2-weighted sequences; however, on T1-weighted sequences, the signal intensity of the fluid may range from hypointense to hyperintense,31,32,35 reflecting the heterogeneous contents. The correlation between MR imaging and the biochemical composition of cyst fluid is complex, with protein, lipid, and iron concentrations having a major influence on cyst signals.32,36 Cyst capsule and solid tumor vividly enhance with contrast.
Noncalcified solid craniopharyngiomas may have CT and MR imaging characteristics that are indistinguishable from those of other pediatric suprasellar neoplasms, including chiasmatic hypothalamic gliomas, germinomas, and pituitary adenomas. Proton MR spectroscopy demonstrates unique spectroscopic profiles that differentiate these tumors.33 Craniopharyngiomas show a dominant peak, consistent with lactate or lipids, and only trace amounts of other metabolites. In contrast, gliomas demonstrate choline, N-acetylaspartate, and creatine, with an increased ratio of choline to N-acetylaspartate compared with that of normal brain; pituitary adenomas show choline peaks or no metabolites at all.
The surgeon’s impression of the extent of tumor resection must be confirmed by neuroimaging. Postoperative imaging with both enhanced MR imaging and CT is best done within 48 hours to avoid the artifacts of surgical trauma.32 Residual tumor should be graded according to the method of Hoffman30: grade 1, no residual tumor or calcification; grade 2, tiny (< 1 mm) fleck of calcification without evidence of enhancement or mass; grade 3, small “calcific chunk” without enhancement or mass effect; grade 4, small contrast-enhancing lesion without significant mass effect; and grade 5, contrast-enhancing mass.
37.4 Clinical Presentation
In children, the slow growth of craniopharyngiomas often results in a delay between the onset of symptoms and diagnosis, with a typical prodrome of 1 to 2 years.37,38 The main presenting signs and symptoms of craniopharyngiomas are related to pressure upon adjacent neural structures.6,9,23,29,37,39–45 Headache from raised intracranial pressure is the most common complaint, occurring in 60 to 75% of cases. Visual symptoms are noted in approximately half of children. Progressive visual loss is often well tolerated by children and not diagnosed until they are noted to be sitting progressively closer to the television. Evidence of hormonal insufficiency, including growth failure, delayed sexual maturation, excessive weight gain, and diabetes insipidus, is present in 20 to 50% of children at diagnosis but is rarely the reason why a child is brought to medical attention. With progressive growth into the frontal lobes and hypothalamus and/or the onset of hydrocephalus, psychomotor slowing, apathy, and short-term memory deficits may also occur, with a decline in academic performance.
Formal preoperative neuro-ophthalmologic, endocrinologic, and neuropsychological evaluations are mandatory. On preoperative testing, 70 to 80% of children will demonstrate abnormal visual acuity or fields.9,23,29,37,39,46 The specific ophthalmologic deficits reflect the direction of growth of the tumor and its compression of various portions of the visual apparatus: prechiasmatic extension will compress the optic nerves, with a loss of visual acuity, whereas posterior tumors will cause chiasmatic compression, with complex visual field defects. Frank papilledema is present in approximately 20% of children.38
Fewer than 30% of children are endocrinologically normal at diagnosis.44,45,47–49 Growth hormone deficiency is the most common finding, present in up to 75% of children. Gonadotropin deficiency is observed in up to 60% of children, and thyroid or adrenal dysfunction in approximately one-third. Diabetes insipidus is relatively uncommon preoperatively, occurring in 9 to 17% of patients.
The essential preoperative endocrine testing includes an evaluation of adrenal function and thyroid function and an assessment of salt and water balance before the initiation of steroid therapy; measurement of gonadotropins and growth hormone is also routinely performed. Failure to preoperatively recognize and correct adrenocorticotropic hormone (ACTH) or thyroid hormone deficiency or appropriately manage diabetes insipidus can result in severe morbidity or death.
37.5 Treatment
Although the optimal treatment of craniopharyngiomas remains controversial, permanent tumor control or cure should be the goal for pediatric craniopharyngiomas. At the center of the debate over potentially curative therapeutic modalities are the extent of surgical excision and the role of cranial irradiation. Total resection of the tumor has been advocated by many centers, whereas others have elected to treat with minimal surgical resection followed by RT. Palliative therapies may provide temporary relief from symptoms; however, progressive solid and cystic tumor growth is inevitable. The management of craniopharyngiomas that have failed primary therapy is associated with significantly increased morbidity and mortality.
Although most physicians would agree that complete removal is desirable for a benign tumor, the tendency of craniopharyngiomas to adhere to adjacent neural tissue and the vessels of the circle of Willis makes excision technically difficult and increases the chance of morbidity and mortality.16,50 In order to avoid perioperative hypothalamic damage, some surgeons prefer to perform a subtotal resection or limited surgery.51,52 Because partial surgery alone will nearly invariably result in tumor recurrence,41,53,54 adjuvant postoperative irradiation is employed to maximize tumor control. Overall, survival rates among patients treated by these two treatment methods have been comparable.9,16,43,51,52,54–59
It is important to point out, however, that the quality of survival after each of these approaches has not been thoroughly documented, even though such information would provide important feedback about the efficacy of these treatment modalities. Much of the follow-up research has focused on physical morbidity, often at the exclusion of the behavioral, emotional, and cognitive sequelae that can negatively impact quality of life.
37.5.1 Surgery
Most pediatric neurosurgeons in North America and Europe favor complete microsurgical resection as the treatment of choice for newly diagnosed craniopharyngiomas.9,12,23,37,41,54,57,60–62 The feasibility and success of radical resection depend on the availability of surgical expertise and postoperative endocrinologic support. They also depend on an understanding of the size and extent of the tumor, whether the tumor is primary or recurrent, the clinical condition of the patient, and the societal resources available to cope with potential postoperative deficits. If the socioeconomic conditions applicable to an individual patient do not provide appropriate long-term endocrinologic support and neurologic care, functional morbidity may overshadow the merits of curative resection.
Proponents of radical surgery argue that the advances in microsurgical techniques have facilitated the treatment of these lesions, and that substitutive therapy can mediate the endocrinologic sequelae secondary to hypothalamic injury. The greater immediate morbidity of this approach may be mitigated by the fact that RT carries risks for unpredictable late neurologic, vascular, and oncogenic side effects, as well as the long-term development of neuropsychological deficits in children within the domains of intelligence, attention, memory, and psychomotor processing speed; the most salient factors increasing these risks are young age at irradiation and total dosage.56,63–66
Operative Technique: Craniotomy
A categorization of the pattern and extent of growth assists in evaluating treatment options and potential surgical approaches, and in predicting outcome. Several different clinical–radiologic classification systems have been proposed9,30,37,38,67; all attempt to describe the degree of vertical and horizontal extension, displacement of the optic nerves and chiasm, number of anatomical regions involved by the tumor, and overall size of the tumor. Size is graded as small (2 cm), medium (2 to 4 cm), large (4 to 6 cm), and giant (> 6 cm)9 (▶ Fig. 37.2). Giant tumors may extend into multiple or all compartments, extending from the medulla to the foramen of Monro.
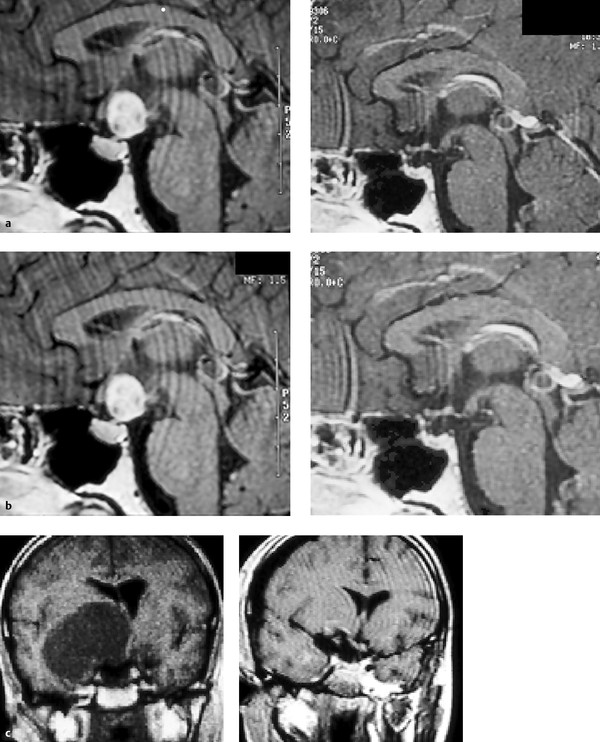
Fig. 37.2 (a) Pre- and postoperative magnetic resonance (MR) images of a small (2 cm) craniopharyngioma. (b) Pre- and postoperative MR images of a medium (4 cm) craniopharyngioma. (c) Pre- and postoperative MR images of a large (5 cm) craniopharyngioma.
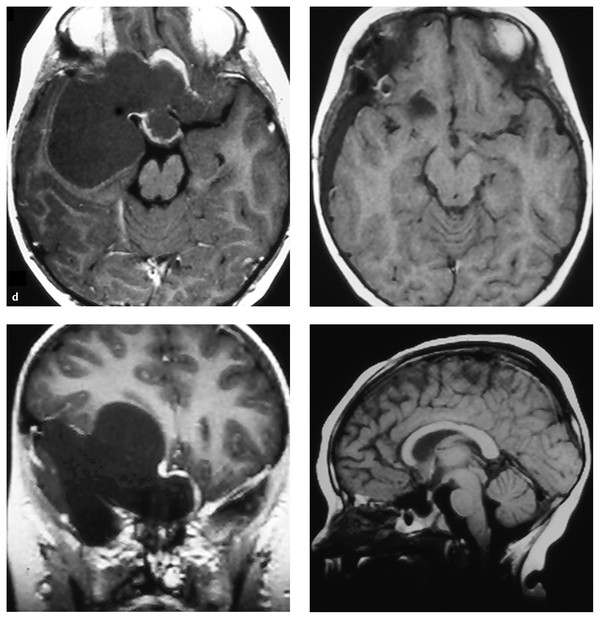
(d) Pre- and postoperative MR images of a giant (7 cm) craniopharyngioma
A variety of operative approaches have been described and championed by different surgeons, including the subfrontal,6,12,28,68 pterional,9,69,70 bifrontal interhemispheric,71,72 subtemporal,73 transcallosal,17 and transsphenoidal approaches.70,74–77 Modified skull base techniques expanding on the pterional approach, including orbitofrontal and orbitozygomatic approaches, have gained popularity over the past two decades.54,62 Surgical adjuncts, including ultrasonic aspirators, frameless stereotaxy, and rigid and flexible neuroendoscopes, should be available and utilized when appropriate.
The senior author prefers a skull base modification of the pterional craniotomy,9 with the additional removal of the supraorbital rim, anterior orbital roof, and zygomatic process of the frontal bone. This approach offers the shortest, most direct route to the suprasellar region and minimizes or eliminates the retraction of normal brain. Tumors extending from the pontomedullary junction (▶ Fig. 37.2d) to above the foramen of Monro can be removed through the pterional approach. In no patient is a cortical resection78 or sacrifice of the olfactory nerve30 necessary.
Dexamethasone (0.1 mg/kg), phenytoin (15 mg/kg), and cephalexin (25 mg/kg) are administered after induction and intubation. Mannitol (0.25 g/kg) is then given at the time of skin incision to help maximize brain relaxation. The diuretic effect is maximal within the first hour of surgery, long before manipulation of the pituitary stalk and hypothalamus may produce the diabetes insipidus that complicates fluid and electrolyte management. Before the dura is opened, either intraoperative ultrasound or frameless stereotaxy is used to determine the location and extent of the tumor and its relationship to the operative exposure.
Throughout the surgery, retraction of the brain is minimized. Mannitol, hyperventilation, and gradual drainage of the CSF through the opened sylvian fissure and basal cisterns will usually provide excellent relaxation, even in the presence of moderate degrees of hydrocephalus. Ventricular drainage is reserved for cases refractory to these maneuvers or when the use of an intraventricular endoscope is anticipated (vide infra). Although hydrocephalus is present in 15 to 66% of patients,9,12,23,37,43,69,72,79 preoperative shunting is reserved for patients with severe symptoms of increased intracranial pressure that is unresponsive to medical management.
Because these tumors often extended diffusely throughout the suprasellar cisterns, displacing and distorting normal structures, identification of the vascular anatomy provides essential landmarks. Starting laterally, the sylvian fissure is widely split, and the distal branches of the middle cerebral artery are identified. The arachnoidal dissection proceeds medially to the main trunk of the middle cerebral artery, which is followed proximally to the ipsilateral carotid bifurcation, anterior cerebral artery, and internal carotid artery. As the carotid is followed proximally to the clinoid, the optic nerve, chiasm, and/or tracts are identified in relation to the tumor.
The premature decompression of a craniopharyngioma, especially a cystic tumor, causes the tumor capsule and arachnoid to become redundant, obscuring the planes of dissection. Working in the prechiasmatic, optic–carotid, and carotid–tentorial triangles, the surgeon develops and maintains an arachnoidal plane between the intact tumor and the branches of the ipsilateral carotid and vessels of the circle of Willis, preserving all of the vessels and their perforating branches. This plane is developed posteriorly until the basilar artery is identified. In primary tumors, the membrane of Liliequist invariably separates the tumor from the basilar artery.
Once the vascular anatomy had been identified and separated from the tumor, the cyst is aspirated and the solid internal component debulked. Care is taken to preserve the capsule of the tumor. Again with the surgeon working in the parachiasmal spaces and maintaining arachnoidal planes, the tumor is progressively dissected free from the optic nerves, the contralateral carotid and its branches, and the inferior aspect of the optic chiasm. An attempt is always made to identify and preserve the pituitary stalk; this can be accomplished in 20 to 30% of the patients. When the stalk cannot be separated free from the tumor, it is sectioned as distally as possible to prevent undo traction on the hypothalamus. After the tumor is dissected free from the entire circle of Willis, the pituitary stalk, and the optic apparatus, the capsule is grasped, and with continuous traction and blunt dissection, the gliotic plane is developed, which allows the tumor to be delivered from its attachment to the hypothalamus in the region of the tuber cinereum. After the tumor is removed, the entire bed must be inspected for inadvertent residual disease. A micromirror or angle endoscope is used to view the undersurface of the chiasm and hypothalamus to confirm a complete resection.
If the tumor extends into the third ventricle or has a significant retrochiasmatic component, the lamina terminalis is fenestrated. The lamina terminalis is easily distinguished from the chiasm, appearing pale, avascular, and often distended by tumor. As retrochiasmatic tumor is removed, the prechiasmatic space may widen, allowing an additional avenue for dissection.
A third ventricular tumor is simultaneously delivered through the lamina terminalis as well as from below the chiasm. Placement of a 2.3-mm neuroendoscope into the lateral or third ventricle assists in monitoring the delivery of the intraventricular component of the tumor. With the endoscope, simultaneous or sequential transcallosal exposure of the intraventricular tumor17 is usually not obligatory.
When the tumor extends into the sella turcica, removal of the posterior planum sphenoidale and tuberculum sellae may be required to gain adequate intrasellar exposure.68 After removal of tumor, any defects communicating with the sphenoid sinus must be obliterated with fat and pericranial grafts.
Operative Technique: Transsphenoidal/Transnasal Surgery
Although most craniopharyngiomas of childhood arise in the region of the tuber cinereum, a small percentage originate from more caudal craniopharyngeal duct cell rests within the sella turcica.75 As these tumors grow, the diaphragma sellae stretches over the dorsal aspect, separating it from suprasellar structures and preventing tumor adherence to the optic apparatus, hypothalamus, and vessels of the circle of Willis. This feature of the pathologic anatomy allows a radical removal of infradiaphragmatic intrasellar tumors through a transsphenoidal/transnasal approach.17,70,74,75,80 From 3 to 15% of pediatric craniopharyngiomas may be amenable to transsphenoidal/transnasal resection.37,81
Transsphenoidal/transnasal surgery in young children may present anatomical difficulties related to the small size of the bony structures and to the lack of a pneumatized sphenoid sinus. The presence of a conchal or pre-pneumatized sphenoid sinus is not a contraindication to transsphenoidal surgery; however, the bone must be meticulously drilled or chiseled under fluoroscopic control to obtain wide access to the sella turcica.75 Thick bones of the sinuses and skull base may require drilling near the sella, planum, and optic nerves. Abe and Lüdecke82 reported that incompletely pneumatized sphenoid sinuses required drilling in 46% of patients in their series of 11 children, but this did not hinder resection in any case. We recommend using an irrigating drill with a diamond bur when the bones of the skull base are drilled to decrease the risk for thermal or mechanical injury to the optic nerves, chiasm, and internal carotid arteries. Other useful adjuncts include a micro-Doppler probe to better identify the carotid artery and stereotactic image guidance with high-resolution CT in addition to standard preoperative MR imaging.
As noted by Im and colleagues,83 the poorly pneumatized sinuses and smaller facial structure of children create an even narrower working corridor. They accomplished gross total resection of six large craniopharyngiomas via the transsphenoidal approach by relying on the cystic nature of all six tumors, the infradiaphragmatic origin of the tumors, and the use of micromirrors for lateral visualization. They noted that predominantly cystic tumors are more common in adults than inchildren, and early decompression can aid in the extirpation of such tumors and removal of the capsule from surrounding structures. With the advent of angled endoscopes and improved optics, micromirrors will likely become obsolete. Nevertheless, taking advantage of the cystic nature of craniopharyngiomas is a critical surgical pearl that facilitates transsphenoidal/transnasal surgery via a long, narrow corridor.
Other technical aspects of the operation do not differ significantly from those for similar surgery in adults, particularly in regard to the overall approach and tumor resection69,70,75,77,84 however, because of the rarity of these tumors, this approach should be utilized only by surgical teams with adequate experience.85
After a wide dural opening, the normal, ventrally displaced pituitary gland is encountered. If the gland obstructs the visualization of the tumor, the pituitary gland should be incised in the midline, then gently pushed laterally to obtain exposure of the dorsally located craniopharyngioma. Once an initial plane of cleavage between the tumor and sellar wall is established, the capsule is opened, with drainage of cyst fluid and debulking of solid neoplasm. Following this internal decompression, the capsule is dissected from the walls of the cavernous sinuses and pituitary gland to complete mobilization of the intrasellar tumor. When the superior capsule adheres to the diaphragma, it must be incised and resected. As the superior craniopharyngioma is delivered, the remaining attachment of the tumor to the pituitary stalk is visualized and detached with bipolar coagulation and sharp dissection to achieve a gross total resection. Resection of the diaphragma invariably produces an intraoperative CSF leak. Obliteration of the sella and sphenoid sinus with a free fat graft is mandatory. Several days of postoperative lumbar drainage is recommended.70
Outcomes of Surgery
Craniotomy
Radiographically confirmed total resection can be accomplished in 80 to 100% of primary tumors in children.9,12,23,37,54,60–62,72 Following radiographically confirmed total resection (Hoffman grade 1 or 2), no adjuvant therapy is administered.86 Accessible tumor demonstrated on postoperative MR imaging or CT that was inadvertently left unresected at primary surgery should be removed. A second operation within several weeks of a primary surgery does not entail any significant added risks or technical difficulty. Recurrence rates following total resection range from 0 to 20% (▶ Table 37.1).9,12,23,37,54,57,60 Most recurrences in children develop within 2 to 3 years.9,12,23,37,60,87–89 Tumor recurrence may bedistant from the primary site as a result of implantation at the time of initial resection,60,90,91 and this has been seen in 7% of recurrent tumors treated by the senior author.92 Reoperation can be curative, especially with solid tumors; however, scarring from previous surgery may increase the technical difficulty of surgery (vide infra).
| Total number of children | Radical surgery | Recurrence | Mortality | |
| Choux et al (1991)37 | 454 | 251 | 19% | 4% |
| Fahlbusch (1997)69 | 30 | 13 | 17% | 0 |
| Hoffman et al (1992)12 | 50 | 45 | 29% | 2% |
| Tomita and McLone (1993)60 | 27 | 23 | 5% | 0 |
| Elliott et al (2010)62 | 57 | 57 | 20% | 3% |
| Yasargil (1996)208 | 61 | 61 | 10% | 2% |
The rates of perioperative mortality following radical surgery have decreased substantially in the last decade, from between 6 and 11% to between 0 and 4%.9,12,22,23,28,37,53,54,60,62,70,79,88,93 The philosophy29 and experience of the surgeon23,57,79 significantly affect the likelihood of achieving a curative total resection with a low incidence mortality or disabling morbidity. Centers performing fewer than two operations for radical resection per year had a good outcome in 52% of cases, compared with 87% of cases for institutions that performed radical surgery more often.57 In addition, the size of the tumor, severity of preoperative deficits, and presence of hydrocephalus all impact on postoperative morbidity,17,79 although not on disease control.94
Younger age has historically been associated with worse outcome, regardless of the therapeutic modality. De Vile et al reported age younger than or equal to 5 years as a predictor of poor outcome after primary surgery and at long-term follow-up, and of a decreased chance of cure.79 Rajan et al also reported a linear trend of improved disease- and treatment-related survival with increasing age in children with craniopharyngioma.16 Erşahin et al noted improved outcome and less chance of recurrence in children older than 10 years of age at the time of diagnosis.95 Several authors have reported higher rates of tumor recurrence in children younger than 5 years.37,79,96,97 However, some studies reported no associations between age and outcome, survival, rates of recurrence,98–101 or obesity and overall health status.102
In the senior author’s experience, radical resection alone at presentation and recurrence offered disease control in 89.5% of 19 patients younger than 5 years with no operative mortality and minimal morbidity.103 One patient developed a fusiform dilatation of the internal carotid artery and experienced a small recurrence that was successfully treated with gamma knife radiosurgery. There was one late mortality in a young child who underwent subtotal resection at New York University after having failed multiple resections, aspirations, and at outside hospitals. Although disease control was not obtained in this patient, death was secondary to an endocrine crisis unrelated to tumor progression. Overall, disease control was successfully achieved in the vast majority of our pediatric patients with surgical resection alone—avoiding the significant risks of irradiation in this young population of patients. Furthermore, we found no difference between the rates of overall survival, neurologic deficits, endocrine deficiency, hypothalamic dysfunction, and quality of life in patients 5 years or younger and those in patients older than 5 years in our entire series of 86 children.
Endocrine disturbances are common after radical resection as a result of hypothalamic manipulation and pituitary stalk sectioning.7,9,37,60,104,105 Endocrine morbidity may be more severe when tumor resection involves bilateral manipulation of the hypothalamus.17 Although a significant percentage of deaths in earlier series were attributable to pituitary insufficiency,105,106 this is uncommon today, provided adequate socioeconomic resources are available.
Hormonal replacement therapy is required in approximately 80% of the children.23,44,47–49,107 Thyroid and cortisol replacement therapy is administered as necessary. Diabetes insipidus is universally present immediately following surgery. Over the course of the first week, diabetes insipidus may alternate with inappropriate antidiuretic hormone release (SIADH). Meticulous attention to fluid balance and electrolyte status is essential to avoid severe fluctuations from hypernatremia to hyponatremia. Permanent diabetes insipidus will develop in approximately 75% of children.9,23,55 Replacement with synthetic vasopressin (DDAVP) provides excellent control of diabetes insipidus in children with an intact thirst mechanism. However, the rare combination of ADH insufficiency and an impaired sense of thirst following aggressive surgery with severe hypothalamic injury remains one of the most complex management problems.104
Excess weight is often the overriding concern during long-term follow-up. As Hoffman and colleages12 have noted, “In a society where fitness and slim bodies are praised, obesity has led to problems with peers.” Weight gain without overt hyperphagia may occur in half of all children undergoing radical surgery, although morbid obesity with lack of satiety is far less common.7,37,44,45,108,109 Many children may become distraught over the alteration in habitus and body image; five of our patients have required individual or family counseling to address these psychological and emotional issues. We now routinely counsel families and older children preoperatively that they may experience a 10 to 15% permanent weight gain. Bilateral hypothalamic damage, particularly in children with larger tumors, may result in an insensitivity to endogenous leptin and a disturbed feedback mechanism from the hypothalamic leptin receptors to the adipose tissue.109 Preoperative weight gain and MR imaging evidence of extensive involvement of the hypothalamus may help predict the patients most at risk for severe postoperative obesity.108
Although most children with craniopharyngiomas are deficient in growth hormone, some will maintain a normal or even accelerated growth rate after surgery, often associated with hyperphagia and obesity.47,48 Normal or accelerated growth following surgery does not indicate the presence of normal growth hormone secretion or ensure continued growth. A complex series of metabolic events, including the activation of insulin-like growth factor-1 by hypothalamic hyperphagia and obesity-induced hyperinsulinemia, may explain this growth pattern.44,110 Many later fail to maintain this growth, and if growth hormone treatment is not instituted, adult height is compromised.47 Growth hormone treatment may be recommended in these children for long-term improved growth velocity, adult height, and other growth hormone–dependent metabolic processes.111 Even when growth hormone replacement may not affect growth, it may help decrease body mass index.112
Total removal of the tumor offers the optimal ophthalmologic recovery and outcome.37 Some degree of deterioration in visual function is present in approximately 20% of children after surgery.37 Maximum improvement in visual acuity and fields is noted within the first postoperative month.46 The extent and duration of preoperative deficits, but not age, are associated with a worse outcome.9,23,37,93 Visual outcome from the authors’ experience and current literature is summarized in ▶ Table 37.2
| Hoffman et al12 (50 children, all primary) | Yasargil et al9 (68 children, 50 primary and 16 recurrent) | Tomita and McLone60 (27 children, all primary) | Elliott et al62 (86 children, 57 primary and 29 recurrent) | |
| Visual field | ||||
| Improved | 39% | 63% | 62% | 26% |
| Stable | 20% | 32% | 19% | 55% |
| Worse | 41% | 5% | 19% | 19% |
| Visual acuity | ||||
| Improved | 55% | 60% | 59% | 16% |
| Stable | 15% | 25% | 30% | 69% |
| Worse | 30% | 15% | 11% | 15% |
Fusiform dilatation of the carotid artery (FDCA) occurs in 10 to 20% of children several months to years following radical surgery,113–115 although it is underreported in the literature. In the senior author’s experience of 76 consecutive children treated with aggressive microsurgical resection of craniopharyngiomas with complete follow-up imaging, almost 10% of children experienced postoperative fusiform aneurysmal dilatation of the supraclinoid carotid artery.115
There have been a total of 26 reported cases of FDCA following craniopharyngioma resection in children and young adults.114–121 The mean age at time of surgery was 10.4 years, the mean tumor size was 3.3 cm, and the mean interval from surgery to the diagnosis of FDCA was 12.5 months. FDCA lesions stabilized in 20 of 24 cases (83%) over an average follow-up duration of 6.5 years (two lesions were treated upon initial diagnosis without serial imaging119,121). All 15 lesions that have been observed for at least 5 years have stabilized in size. Only two (7.7%) cases became symptomatic (headache in one patient, vision loss from optic nerve compression in another patient). No patient experienced rupture during the follow-up period.
The pathogenesis is unclear but most likely is related to operative manipulation and retraction causing injury to the vasa vasorum and subsequent weakening of the muscular layer. Although RT may have contributed to the onset or progression of FDCA in three reported cases, the majority of patients (88%) had not received RT before the onset of FDCA. The natural history of FDCA appears to be dramatically different from that of radiation-induced aneurysms,122–126 most of which are saccular in morphology, not fusiform. Postradiation aneurysms occurred at a mean of 10 years following treatment and presented with subarachnoid hemorrhage in over 60% of cases. The putative pathogenesis of RT-induced aneurysms is endothelial damage from the ionizing radiation; smaller vessels and capillaries are usually more affected than larger-caliber vessels.127
The pathogenesis and natural history of radiation-induced aneurysms are markedly different from those of FDCA following craniopharyngioma surgery and should not be treated in a similar manner. Our experience is consistent with that of most other authors,116,120,128 that FDCA may be a relatively frequent but clinically benign complication following craniopharyngioma and should be radiographically observed. If operation is required for recurrent tumor, Sutton has recommended approaching the tumor from the opposite side.114 For small, recurrent craniopharyngiomas with FDCA, stereotactic radiosurgery may be a useful and safe option, with high rates of reported local control.129–132 Direct reconstruction of the carotid artery is dangerous and should be avoided. Whether wrapping the dilatation has long-term benefit is similarly uncertain.113,114 Conservative follow-up with MR imaging and MR angiography is appropriate.
Transsphenoidal Resection
Total resection can be accomplished in 60 to 90% of primary infradiaphragmatic intrasellar craniopharyngiomas; however, the rate of success drops to 10 to 60% for recurrent tumors.69,70,76,77,81,84 In experienced hands, operative mortality ranges from 0 to 4% and nonendocrine morbidity from 15 to 25%, with children tending to do better than adults.69,70,75,77,81 The incidence of new diabetes insipidus, but not of other endocrine deficiencies, appears to be less than with transcranial surgery.17,69,70,84 Impairment of psychosocial function is uncommon.69,70 Recurrence after total resection of a primary tumor develops in 0 to 43% of patients, with the incidence of recurrence substantially less in the most experienced centers.69,70,76,81,84
A recent meta-analysis of the major surgical series for the treatment of pediatric craniopharyngiomas demonstrated that the patients treated with transsphenoidal/transnasal approaches had excellent outcomes.133 Compared with the children who had formal craniotomies, the patients treated with transsphenoidal/transnasal surgery had better visual, neurologic, endocrinologic, and oncologic outcomes.
Directly comparing outcomes following craniotomy and transsphenoidal/transnasal surgery for pediatric craniopharyngioma may not be valid. Critical to this analysis is the identification of baseline differences between the populations of patients selected for each surgical approach (▶ Table 37.3). Craniopharyngiomas treated transsphenoidally tended to be smaller, more often completely or predominantly intrasellar, and often cystic in nature.70,134,135 These patients also had less hydrocephalus and no reported instances of elevated intracranial pressure.
| Variable | Transcranial series, % (No.) | Transsphenoidal Series, % (No.) | p Value |
| Preoperative vision deficits | 53.5% (1,051 of 1,966) | 68.5% (124 of 181) | < 0.0001 |
| Preoperative hydrocephalus | 41.7% (678 of 1,625) | 5.1% (5 of 99) | < 0.0001 |
| Preoperative elevated ICP | 42.6% (729 of 1,713) | 0% (0 of 138) | < 0.0001 |
| GTR | 60.9% (1,693 of 2,780) | 72.1% (199 of 276) | 0.0003 |
| Recurrence after GTR | 17.6% (261 of 1,518) | 8.0% (16 of 201) | 0.0005 |
| Operative mortality | 2.6% (68 of 2,622) | 1.3% (5 of 373) | 0.21 |
| Neurologic morbidity | 9.4% (200 of 2,140) | 3.1% (10 of 325) | < 0.0001 |
| Diabetes insipidus | 69.1% (1,437 of 2,076) | 23.9% (76 of 318) | < 0.0001 |
| Vision improvement | 47.7% (454 of 1,051) | 85.5% (106 of 124) | < 0.0001 |
| Vision deterioration | 13% (263 of 2,029) | 2.3% (8 of 352) | < 0.0001 |
| Obesity or hyperphagia | 32.2% (439 of 1,363) | 32.1% (35 of 109) | 1.00 |
| Overall survival | 90.3% (2039 of 2258) | 93.9% (216 of 230) | 0.075 |
| Abbreviations: GTR, gross total resection; ICP, intracranial pressure. Source: From Elliott RE, Jane JA Jr, Wisoff JH. Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 2011;69(3):630–643, discussion 643.133 | |||
Prior studies have reported worse outcomes in patients with hydrocephalus, larger tumors, and poor preoperative functional status.9,62,79,136 Moreover, large tumor size has been associated with increased operative mortality,9 neurologic and hypothalamic morbidity,9,79,95 a lower probability of gross total resection,61,79,135 and higher recurrence rates.79,95,137,138 The selection bias due to these baseline differences may explain the improved outcomes in the transsphenoidal surgery group in the meta-analysis. Regardless of these findings, further experience with transsphenoidal/transnasal approaches in conjunction with innovations in endoscopic instrumentation will undoubtedly continue to expand the indications for transsphenoidal/transnasal surgery and, ultimately, limit patient morbidity and improve overall outcomes and quality of life. ▶ Table 37.4 summarizes the relative indications, advantages, and disadvantages of the transcranial and transsphenoidal/transnasal approaches.
| Transcranial approaches | Transsphenoidal approaches | |
| Indications | Large or gigantic tumors | Purely intrasellar tumors |
| Cystic or solid suprasellar tumors | Intra- and suprasellar subdiaphragmatic tumors | |
| Tumor entirely within the third ventricle | Predominantly cystic tumors | |
| Advantages | Excellent visualization and control of major arteries | Early decompression of tumor before manipulation of optic apparatus |
| Shorter hospital stays | ||
| Less tissue trauma and brain retraction | ||
| Less pain | ||
| Contraindications | Intrasellar tumors | Tumors with extension lateral to the internal carotid artery |
| Large or gigantic tumors | ||
| Encasement of arteries of the circle of Willis or optic apparatus | ||
| Suprasellar lesions with peripheral calcifications | ||
| Disadvantages | Brain retraction | Immature (conchal) sphenoid sinus |
| Manipulation of optic apparatus before tumor removal | Poor control of hemorrhage (arterial injury) | |
| Risk for cerebrospinal fluid leak | ||
| Source: From Elliott RE, Jane JA Jr, Wisoff JH. Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 2011;69(3):630–643, discussion 643.133 | ||
37.5.2 Irradiation
Despite the fact that aggressive surgery is often considered the primary treatment for craniopharyngiomas, there is a long history of employing RT in the treatment of these tumors. As early as the first half of the 20th century, RT was considered useful in the treatment of craniopharyngiomas.100,139 Radiation has been utilized as primary treatment following cyst aspiration, biopsy, or subtotal resection, and as salvage in the setting of recurrent disease. Kramer et al reported a series of 10 consecutive patients treated at the Royal Marsden Hospital, London, England, during the early 1950s with biopsy and cyst decompression followed by radiation.101,140 Doses of 55 to 70 Gy (gray) were delivered over 6 to 7 weeks with 2 million-volt roentgen rays. At 5 to 15 years of follow-up, three of the adult patients had died of unrelated causes. The remaining adults and the six children were alive without sign of recurrence, and all were considered to be “functioning well.”
Subtotal resection alone results in progression rates of 55 to 85%, as assessed in several retrospective series.16,59,101,141,142 Progression often occurs early in this setting, within months to a few years after subtotal resection.5,101 The addition of postoperative RT in the setting of gross residual disease can decrease that recurrence rate to 15 to 20% (▶ Table 37.5). Although no Phase III data exist for a direct comparison of subtotal resection alone versus subtotal resection followed by RT, the abundant retrospective data, although imperfect, demonstrate improved local control with the addition of RT following the subtotal resection of tumor.
| Series | Years of study | Subtotal resection | Subtotal resection + RT |
| UCSF209 | 1956–1972 | 5/9 (55%) | 2/10 (20%) |
| University of Iowa59 | 1961–1986 | 17/20 (85%) | 0/8 (0%) |
| CHOP101 | 1974–2001 | 7/9 (78%) | 3/18 (16%) |
| St. Jude52 | 1984–2001 | 6/7 (86%) | 2/15 (13%) |
| Abbreviations: CHOP, Children’s Hospital of Philadelphia ; RT, radiotherapy; UCSF, University of California, San Francisco. | |||
RT has also been shown to be efficacious in the setting of recurrent disease. Relatively modern series show effective salvage of patients with recurrent disease,52,55,101,143,144 and it appears that the use of RT at the time of recurrence or progression yields local control rates similar to those achieved when RT is used in the early (1 to 3 months) postoperative period (▶ Table 37.6). Furthermore, overall survival remains high (85 to 100%) in the salvaged groups.
| Series | Years | Early (No. of patients) | Delayed (No. of patients) |
| JCRT55 | 1970–1990 | 82% local control (37) | 83% local control (6) |
| CHOP101 | 1974–2001 | 84% local control (18) | 83% local control (22) |
| St. Jude52 | 1984–2001 | 86% local control (15) | 87% local control (8) |
| Christie Hospital143 | 1976–2002 | 79% 10-year PFS (42) | 77% 10-year PFS (44) |
| University of Seoul144 | 1985–2002 | 91.2% 10-year PFS (25) | 91.3% 10-year PFS (25) |
| Abbreviations: CHOP, Children’s Hospital of Philadelphia; JCRT, Joint Center for Radiation Therapy; PFS, progression-free survival | |||
Given the ability of RT to effectively salvage recurrence and maintain high overall survival rates, the argument could be made to postpone RT until the time of recurrence. However, given a median time to progression of only 12 months in patients with subtotal resection,142 there may not be much advantage gained with this approach except in young children, who may benefit from a delay that allows further brain development. These children should be followed closely with MR imaging and treated with irradiation at the first sign of radiographic progression.
Dose and Volume
It is not clear if there is a dose–response relationship for craniopharyngiomas. Three series have noted a dose response for local control with doses higher than 54 to 60 Gy.56,145,146 However, the vast majority of series in the literature do not report a dose response,59,147 and the reported high local control rates have been achieved with doses of 54 to 55.8 Gy.52,55,101 Given the potential increased risks for optic neuropathy and necrosis with doses of 60 Gy or more, most radiation oncologists use 54 to 55.8 Gy (in daily fractions of 1.8 Gy) in the treatment of craniopharyngiomas.
Treatment planning for RT relies on the accurate delineation of tumor on CT and MR imaging. The volume of RT is defined as the tumor with a margin. It is important to include the full extent of the cystic components of the tumor in the treatment volume. If cysts have been drained, the target volume should be based on the decompressed volume, including the full extent of the cyst wall. Thus, the gross tumor volume usually includes any radiographically identified enhancing or nonenhancing tumor and cyst/cyst wall. This classically has been expanded by 10 mm to create a clinical target volume; however, some authors have suggested that with highly conformal techniques, tight immobilization, and real-time imaging, the clinical target volume margins might even be decreased to 5 mm,148 and most radiation oncologists favor the smaller expansions. An additional 3 to 5 mm is added to the clinical target volume to create a planning target volume.
As planning target volumes have become increasingly conformal, the importance of monitoring for cyst expansion and adaptive planning needs to be emphasized. It has been reported that 35 to 60% of patients may manifest an increase in the target volume during irradiation,148,149 and the increase in planning target volume on average is 11%.148 In the early experience with conformal RT for craniopharyngiomas at St. Jude Children’s Research Hospital, however, few patients required a change in treatment planning despite the change in volume during irradiation, primarily because relatively “large” clinical target volume and planning target volume margins of 10 mm and 3 to 5 mm, respectively, were used. As margins have become “tighter,” serial imaging during therapy should be considered to ensure that there is no re-expansion of cysts that would require altering the target volume and/or adjusting the treatment plan. Unfortunately, it is still unclear how to identify patients who are at risk for tumor expansion during RT, and the best method for imaging during RT has not yet been defined.
Techniques of External Beam Radiation
A variety of techniques have been employed in the treatment of craniopharyngiomas. In the 1950s and 1960s, treatment was delivered with cobalt 60, usually through lateral parallel opposed portals. This meant that both temporal lobes received higher doses than did the tumor. The advent of linear accelerators changed the way radiation was delivered, allowing a “third” field or “arc rotation” such that the doses to the surrounding normal brain could be decreased. The advent of CT and MR imaging has allowed improved definition of tumor volumes and normal structures at risk, and increasingly sophisticated computer software has advanced treatment planning and delivery.
Conformal Radiation Therapy and Intensity-Modulated Radiation Therapy
A typical three-dimensional conformal RT plan might employ five fields to deliver the full dose to the target volume while limiting the dose to the surrounding brain to 30 to 40% of the target volume dose (▶ Fig. 37.3). Published data support the idea that treating conformally to small volumes results in outcomes equivalent to those achieved with conventionally planned RT. Merchant et al showed in a Phase II trial of conformal RT that the irradiated volume for craniopharyngioma could be safely reduced without compromising tumor control.150 Doses of 54 to 55.8 Gy were administered to the gross tumor volume (solid and cystic components), and a 10-mm margin was added to create a clinical target volume. The estimated 3-year progression-free survival rate was 90%.
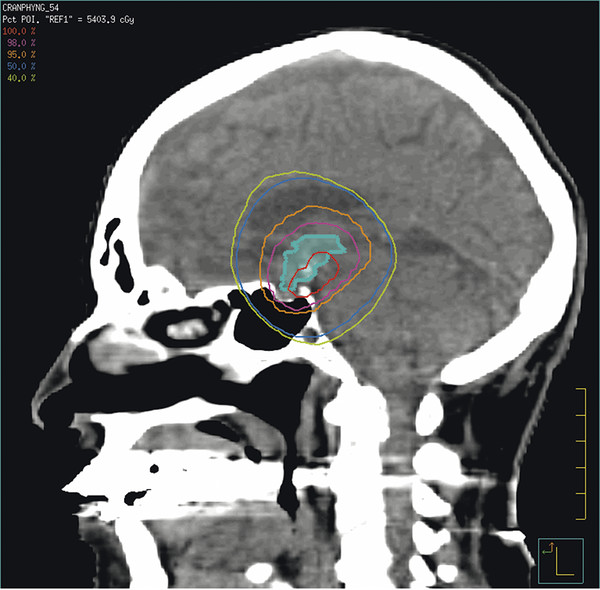
Fig. 37.3 Three-dimensional conformal radiation therapy treatment plan.









