Chapter 4 Daytime Sleepiness and Alertness
Epidemiology of Sleepiness
Sleepiness in Limited Populations or Populations of Convenience
In surveys of relatively small, selected populations, 0.3% to 36% of respondents reported excessive sleepiness. Surveys with reported excessive sleepiness rates of less than 3% generally are from earlier studies that focused on hypersomnia.1 In later studies, in which 4% to 9% rates were reported, more specific questions about excessive sleepiness during the day or relative to one’s peers were asked.2 In some surveys, postprandial, or midday, sleepiness was distinguished from sleepiness at other times of the day, a distinction discussed later in regard to the circadian correlates of sleepiness. Somewhat higher rates are reported in other selected populations using sleepiness scales. For example, the Epworth Sleepiness Scale (ESS), a scale that requires one to estimate the likelihood of falling asleep in different situations, was completed by 740 day workers in 8 industrial plants in Israel and 23% of respondents had ESS scores indicative of excessive sleepiness (i.e., scores >10).3
Prevalence rates for sleepiness of 15% and greater also have been found for specific age groups that are consistent with smaller laboratory studies using the physiological measure of sleepiness, the Multiple Sleep Latency Test (MSLT), which is described later. Young adults were sleepier, on average, than a comparison group of middle-aged adults, and about 20% of the young adults had mean daily sleep latencies of less than 5 minutes, a level of sleepiness considered pathological.4 Healthy elderly also were found to be physiologically sleepier than middle-aged adults.5 In surveys of the work force engaged in shift or night work, complaints of excessive sleepiness during waking hours are more frequent than among day workers, and continuous ambulatory electroencephalographic (EEG) field monitoring has confirmed the sleepiness.6
Sleepiness in Representative Populations
Representative survey studies of national populations have been done. In a study representative of the Finnish population, 11% of women and 7% of men reported daytime sleepiness almost every day.7 In another survey, representative of a large geographical area in Sweden, 12% of respondents thought their sleep was insufficient.8 In that survey, insufficient sleep, and not its consequent daytime sleepiness, was the focus of the questions. Two studies representative of the United States population used the MSLT to assess sleepiness. Given the necessary time commitment required of participants in MSLT studies, the representative integrity of study results is critically dependent on the recruitment response rate. From a large southeastern Michigan random sample (n = 1648) representative of the U.S. population, a subsample (n = 259) with a 68% response rate was recruited to undergo a nocturnal polysomnogram (NPSG) and MSLT the following day. The prevalence of excessive sleepiness, defined as a MSLT average sleep latency of less than 6 minutes, was 13%.9 In another probability sample of 6,947 Wisconsin state employees, a subsample (n = 632), collected with a 52% response rate, slept at home and then completed a MSLT in the laboratory the next day. Twenty-five percent had an average sleep latency of less than 5 minutes.10 These two studies also used the ESS to assess sleepiness; in the Michigan study 20% had ESS scores greater than 10, and in the Wisconsin study 25% had scores greater than 11. The higher prevalence in the Wisconsin study, despite the more stringent definition of sleepiness (MSLT of 5 vs. 6 minutes and ESS of 11 vs. 10), could be attributed to an age difference in the samples (51 vs. 42 yr on average) or the previous night’s sleep time and circumstances (habitual at home, on average 7.1 hr vs. standard laboratory 8.5 hr). In Figure 4-1 the distribution of sleepiness, defined as average sleep latency on the MSLT, is illustrated for the Michigan population representative sample. The average sleep latency of various clinical samples and experimental sleep time manipulations is provided for comparisons.
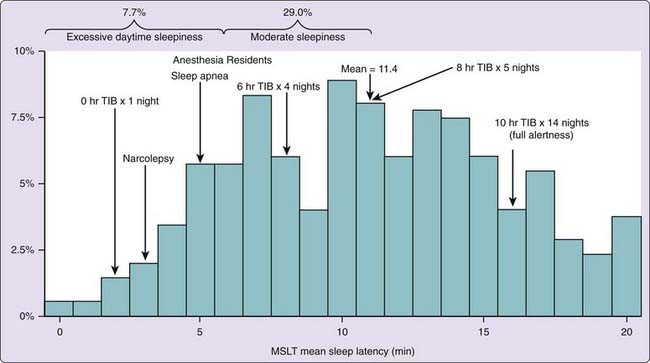
Figure 4-1 The distribution of mean daily sleep latency (min) on the multiple sleep latency test in a subsample (n = 259) recruited (68% response rate) from a large Southeastern Michigan random sample (N = 1648) representative of the U.S. population. The population mean is 11.4 minutes and this is compared to means reported for various patient groups60,70,71 and the means found in healthy normals after various bedtime manipulations.23,62 TIB, time in bed.
Risk Factors for Sleepiness
The risk factors for sleepiness identified in the various surveys includes hours of daily sleep, employment status, marital status, snoring, and depression. Among 26- to 35-year-old members of a large health maintenance organization in Michigan, respondents reported 6.7 hours of sleep on weekdays and 7.4 hours on weekend days, on average.11 The hours of sleep were inversely related to daytime sleepiness scores on the Sleep–Wake Activity Inventory (SWAI). Both these variables were related to employment and marital status, with full employment and being single predictive of less sleep time and more sleepiness. Self-reported snoring and depression, as measured by a structured diagnostic interview, were also associated with increased sleepiness. In the Finnish study cited earlier, sleepiness was associated with moderate to severe depression and with snoring more than three times per week.7
Nature of Sleepiness
Physiological Need State
Sleepiness, according to a consensus among sleep researchers and clinicians, is a basic physiological need state.12 It may be likened to hunger or thirst, which are physiological need states basic to the survival of the individual organism. The presence and intensity of this state can be inferred by how readily sleep onset occurs, how easily sleep is disrupted, and how long sleep endures. Deprivation or restriction of sleep increases sleepiness, and as hunger or thirst is reversible by eating or drinking, sleep reverses sleepiness. In the organism’s daily homeostatic economy, severe deprivation states do not normally occur and hence are not routinely responsible for regulating eating or drinking; other factors (i.e., taste, smell, time-of-day, social factors, biological variables) modulate these behaviors before severe deprivation states develop. Similarly, routine consumption of sleep is not purely homeostatic, but is greatly influenced by social (i.e., job, family, and friends) and environmental (i.e., noise, light, and bed) factors.
The subjective experience of sleepiness and its behavioral indicators (yawning, eye rubbing, nodding) can be reduced under conditions of high motivation, excitement, exercise, and competing needs (e.g., hunger, thirst); that is, physiological sleepiness may not necessarily be manifest. The expression of mild to moderate sleepiness can be masked by any number of factors that are alerting, including motivation, environment, posture, activity, light, or food intake. Studies have shown that average sleep latency on the MSLT is increased by 6 minutes when sitting versus lying in bed and also by 6 minutes when immediately preceded by a 5-minute walk.13 However, when physiological sleepiness is most severe and persistent, the ability to reduce its impact on overt behavior wanes. The likelihood of sleep onset increases and the intrusion of microsleeps into ongoing behavior occurs. On the other hand, a physiologically alert (sleepiness and alertness are used here as antonyms) person does not experience sleepiness or appear sleepy even in the most soporific situations. Heavy meals, warm rooms, boring lectures, and the monotony of long-distance automobile driving unmask physiological sleepiness when it is present, but they do not cause it.
Adaptation to the chronic experience of sleepiness most probably occurs. Clinicians have reported anecdotally that successfully treated patients will frequently comment that they had forgotten the experience of complete alertness. Reduced sensitivity to chronic sleepiness is a likely explanation for the disparities between subjective assessments, even when done with validated scales and the MSLT.5,14 Typically, it is the most sleepy individuals that show the greatest disparity in subjective versus objective assessments.5,14 Such individuals deny sleepiness despite significant objective indicators of sleepiness. On the other hand, basally alert individuals (ESS mean 5.6, SEM 0.3) after a one night acute sleep restriction were quite accurate in estimating their sleepiness relative to the increases in EEG theta activity shown during a simulated driving task.15 Studies have also shown that compensation occurs to the cognitive and behavioral effects of experimental sleep restriction and increased sleepiness, particularly when the sleep loss is mild and accumulates at a slow rate.16 The absence of a readily apparent behavioral deficiency probably also contributes to the subjective-objective disparity seen in chronically sleepy individuals. Finally, findings from a general population study suggest that subjective sleepiness has multiple dimensions, beyond an increased tendency to fall asleep.17 Consequently, patients often mistake chronic debilitating fatigue for sleepiness.18
The specific nature of this physiological need state is unclear. Whether sleepiness is one-dimensional, varying only in severity, or multidimensional, varying as to etiology or chronicity, has been discussed.19 If it is one-dimensional, whether or not sleepiness and alertness are at opposite poles of the dimension is also an issue. Earlier, it was noted that sleepiness and alertness are being used as antonyms, which suggests a unipolar state. However, it is possible that sleepiness varies from presence to absence and is distinct from alertness. It was noted that sleepiness may be multidimensional, and among the different types of sleepiness cited are rapid eye movement (REM) versus non–rapid eye movement (NREM) and core versus optional sleepiness.19 A complete discussion of the heuristic value and evidence to support these distinctions is beyond the scope of this chapter. Nonetheless, the point must be made that these theoretical perspectives may be colored by different measures, experimental demands, populations studied, and subject or patient motivations (i.e., sensitivity to and capacity to counteract sleepiness).
Neural Substrates of Sleepiness
The substrates of sleepiness have yet to be determined. It is assumed that sleepiness is a central nervous system (CNS) phenomenon with identifiable neural mechanisms and neurochemical correlates. Various electrophysiological events suggestive of incipient sleep processes appear in behaviorally awake organisms undergoing sleep deprivation. In sleep-deprived animals, ventral hippocampal spike activity, which normally is a characteristic of NREM sleep, increases during behavioral wakefulness and in the absence of the usual changes in cortical EEG indicative of sleep.20 Human beings deprived, or restricted, of sleep show identifiable microsleep episodes (brief intrusions of EEG indications of sleep) and increased amounts of alpha and theta activity while behaviorally awake.21 The evidence suggests that these electrophysiological events are indicants of sleepiness.
An emerging literature of neuroimaging studies, both structural and functional, have suggested specific brain systems that may be involved in sleepiness. Sleep deprivation in young healthy volunteers reduced regional cerebral glucose metabolism, as assessed by positron emission tomography, in thalamic, basal ganglia, and limbic regions of the brain.22 Functional magnetic resonance imaging (fMRI) after chlorpheniramine (a sedating antihistamine) compared with placebo showed increased frontal and temporal activation.23 Because fMRI was conducted while the subject was performing cognitive tasks, the authors interpreted the observed brain activation to have resulted from the increased mental effort, due to sleepiness, required to perform the task. Two groups of patients with severe or slight hypersomnia associated with paramedian thalamic stroke on an MRI showed lesions involving dorso- and centromedial thalamic nuclei, bilateral lesions in the severe group and unilateral in the slight group.24 As yet, these imaging data are not conclusive. They do suggest it may be possible to identify brain regions and functions that vary with sleepiness. But the nature of the alteration may depend on the behavioral load imposed on the sleepy subject as well as the cause of the sleepiness.
The neurochemistry of sleepiness and alertness involves critical and complex issues that have not yet been fully untangled (see Chapters 18, 37, 42, and 44). First, a basic issue concerns whether sleepiness and alertness have a neurochemistry specific and unique from that associated with the sleep process, per se. Second, it is not clear whether sleepiness and alertness are controlled by separate neurochemicals or by a single substance or system. Third, the relation of the neurochemistry of sleepiness and alertness to circadian mechanisms has not yet been determined. Given the number of questions, it should be of no surprise that these are areas of active research.
Neurophysiological studies of sleep and wake mechanisms have implicated histamine, serotonin, the catecholamines, and acetylcholine in control of wake and gamma-aminobutyric acid (GABA) for sleep.25 Evidence from animal studies is emerging that suggests extracellular adenosine is the homeostatic sleep factor, with brain levels accumulating during prolonged wakefulness and declining during sleep.26 The peptide hypocretin/orexin has received much attention for its role in the pathophysiology of narcolepsy.27 It is considered to be a major wake-promoting hypothalamic neuropeptide and a hypocretin/orexin deficiency has been found in human narcolepsy. Its interactive role in the homeostatic control of sleep and sleepiness has yet to be determined. It is discussed in greater detail in Chapters 18, 37, 42, and 44.
Pharmacological studies provide other interesting hypotheses regarding the neurochemistry of sleepiness-alertness. For example, the benzodiazepines induce sleepiness and facilitate GABA function at the GABAA receptor complex, thus implicating this important and diffuse inhibitory neurotransmitter.25 Another example involves histamine, which is now considered to be a CNS neurotransmitter and is thought to have CNS-arousing activity.25 Antihistamines that penetrate the CNS produce sleepiness.28 A functional neuroimaging study of histamine H1 receptors in human brain found that the degree of sleepiness associated with cetirizine (20 mg) was correlated to the degree of H1 receptor occupancy.29
Stimulant drugs suggest several other transmitters and neuromodulators. The mechanism of action of one class of drugs producing psychomotor stimulation and arousal, the amphetamines, is blockade of catecholamine uptake.30 Another class of stimulants, the methylxanthines, which include caffeine and theophylline, are adenosine receptor antagonists. Adenosine, considered the key neurochemical in the homeostatic regulation of sleep, has inhibitory activity on the two major excitatory neurotransmitters acetylcholine and glutamate. It may be a biomarker of sleepiness.26 On the other hand, some contradictory evidence limits making definitive conclusions. The space here is too limited to discuss all the evidence in detail. In conclusion, although it is widely held that sleepiness is a physiological state, its physiological substrates are as yet not fully defined.
Assessment of Sleepiness
Quantifying Sleepiness
Assessment problems were evident early in research on the daytime consequences of sleep loss. Sleep loss compromises daytime functions; virtually everyone experiences dysphoria and reduced performance efficiency when not sleeping adequately. But a majority of the tasks used to assess the effects of sleep loss are insensitive.31 In general, only long and monotonous tasks are reliably sensitive to changes in the quantity and quality of nocturnal sleep. An exception is a 10-minute visual vigilance task, completed repeatedly across the day, during which lapses (response times greater than or equal to 500 milliseconds) and declines in the best response times are increasingly observed as sleep is lost, either during total deprivation or cumulatively over nights of restricted bedtimes.32
In various measures of mood, including factor analytic scales, visual analogue scales, and scales for specific aspects of mood, subjects have shown increased fatigue or sleepiness with sleep loss. Among the various subjective measures of sleepiness, the Stanford Sleepiness Scale (SSS) is the best validated.33 Yet clinicians have found that chronically sleepy patients may rate themselves alert on the SSS even while they are falling asleep behaviorally.34 Such scales are state measures that query individuals about how they feel at the present moment. Another perspective is to view sleepiness behaviorally, as in the likelihood of falling asleep, and thus ask individuals to rate that likelihood in different social circumstances and over longer periods. The Epworth Sleepiness Scale (ESS) has been validated in clinical populations showing a 74% sensitivity and 50% specificity relative to the MSLT in a study of sleep disorders patients.35 It asks about falling asleep in settings in which patients typically report falling asleep (e.g., while driving, at church, in social conversation). The time frame over which ratings are to be made is 2 to 4 weeks.
The standard physiological measure of sleepiness, the MSLT, similarly conceptualizes sleepiness as the tendency to fall asleep by measuring the speed of falling asleep. The MSLT has gained wide acceptance within the field of sleep and sleep disorders as the standard method of quantifying sleepiness.36 Using standard polysomnographic techniques, this test measures, on repeated opportunities at 2-hour intervals throughout the day, the latency to fall asleep while lying in a quiet, dark bedroom. The MSLT is based on the assumption, as outlined earlier, that sleepiness is a physiological need state that leads to an increased tendency to fall asleep. The metric typically used to express sleepiness has been average daily sleep latency (i.e., mean of the four or five tests conducted), but survival analyses have also been successfully used.37 The reliability and validity of this measure have been documented in a variety of experimental and clinical situations.38 In contrast to tests of performance, motivation does not seem to reduce the impact of sleep loss as measured by the MSLT. After total sleep deprivation, subjects can compensate for impaired performance, but they cannot stay awake long while in bed in a darkened room, even if they are instructed to do so.39
An alternative to the MSLT, suggested by some clinical investigators, is the Maintenance of Wakefulness Test (MWT). This test requires that subjects lie in bed or sit in a chair in a darkened room and try to remain awake.40 Like the MSLT, the measure of ability to remain awake is the latency to sleep onset. The test has not been standardized: there are 20-minute and 40-minute versions, and the subject is variously sitting upright in a chair, lying in bed, or semirecumbent in bed. The reliability of the MWT has not been established either. One study reported sensitivity to the therapeutic effects of continuous positive airway pressure (CPAP) in patients with sleep apnea,41 and several studies reported sensitivity to the therapeutic effects of stimulants in narcolepsy.42 A study attempted to tease apart the critical factors being measured by the MWT and concluded that, unlike the MSLT which measures level of sleepiness, the MWT measures the combined effects of level of sleepiness and the degree of arousal as defined by heart rate.43
Relation of Sleepiness to Behavioral Functioning
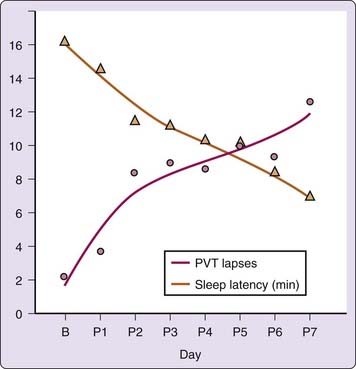
Given that the MSLT is a valid and reliable measure of sleepiness, the question arises as to how this measure relates to an individual’s capacity to function. Direct correlations of the MSLT with other measures of performance under normal conditions have not been too robust. Several studies have found, however, that when sleepiness is at maximum levels, correlations with performance are high. For example, MSLT scores after sleep deprivation,44 after administration of sedating antihistamines,45 and after benzodiazepine administration46 correlate with measures of performance and even prove to be the most sensitive measure. Studies also have compared levels of sleepiness to the known performance-impairing effects of alcohol.47 A study relating performance lapses on a vigilance task to the cumulative effects of sleep restriction found a function comparable to that of the MSLT under a similar cumulative sleep restriction (Fig. 4-2).48 The reason many studies have found weak correlations between performance and MSLT at normal or moderate levels of sleepiness is that laboratory performance and MSLT are differentially affected by variables such as age, education, and motivation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree