Chapter 105 Clinical Features and Evaluation of Obstructive Sleep Apnea and Upper Airway Resistance Syndrome
Abstract
Obstructive sleep apnea (OSA) syndrome and the related upper airway resistance syndrome (UARS) are becoming increasingly recognized as leading causes of daytime sleepiness. Together they are referred to as sleep-disordered breathing (SDB). When apneas and hypopneas occur with a specified frequency during sleep and in conjunction with symptoms such as daytime somnolence, the term obstructive sleep apnea is applied,1 rather than the older terminology obstructive sleep apnea–hypopnea (OSAH).
Obstructive apnea is defined as complete or near-complete cessation of airflow for a minimum of 10 seconds; obstructive hypopnea has been defined differently depending on the author and the type of equipment used to monitor nasal airflow. None of the currently available sleep scoring systems have adequately resolved the controversies surrounding the scoring criteria for an obstructive hypopnea. The American Academy of Sleep Medicine guideline1 for scoring an obstructive hypopnea recommends scoring the event based on the system used to monitor airflow at the nose, and this recommendation is based on consensus rather than objective data. The number of abnormal breathing events during nocturnal sleep is presented as apnea–hypopnea index (AHI), reflecting the number of apneas plus hypopneas per hour of sleep. Due to the variability of what is being measured, the term respiratory disturbance index (RDI) sometimes is used interchangeably and incorrectly with AHI; RDI refers to the number of apneas plus hypopneas plus respiratory effort–related arousals (RERAs) per hour of sleep.
Epidemiology
OSA was first recognized during polysomnographic monitoring in severely obese patients with the pickwickian syndrome.2 Epidemiologic studies in the United States performed in the 1990s based on the general population or community-based cohorts consisting of primarily white persons between 30 and 60 years of age estimated the prevalence of OSA (defined by AHI ≥ 5) to be 24% in men and 9% in women. When complaints of sleepiness were taken into account, 4% of men and 2% of women had OSAS.3–9 More-recent studies after the explosion of the obesity epidemic in industrialized countries have changed these results. The incidence of OSA in whites in countries affected by the obesity epidemic has reached approximately 8%. Studies in other ethnic groups are also available. In Hong Kong the prevalence in the late 1990s was reported to be similar to the one seen in whites (4.1% in middle-aged men and 2.1% in women). A study of Chinese subjects in Singapore reported an estimated prevalence of 15%, and the prevalence was reported to be 7.5% in urban middle-aged Indian men, 4.5% in Korean men and 3.2% in middle-aged Korean women, and 8.8% in Malaysian men and 5.1% in Malaysian women.10–12 The prevalence of OSA increases with aging.13,14
Obesity might also contribute the increased prevalence of OSA. In addition, techniques used to calculate prevalence might play a role in discrepancies between studies. The latest study performed on a representative sample of specific districts in the city of Sao Paulo from 2007 to 2008 is the only study in which more than 1100 participants were monitored with actigraphy for 1 week, underwent 1 night of polysomnography, answered multiple questionnaires, and had a complete physical evaluation. This Sao Paulo study examined a sample that is similar to residents from the midwestern United States, in particular with obesity, and found a prevalence for OSA to be about 15%.15 The prevalence of OSA is thus variable depending on geographic location, given that age and the degree of obesity also contribute to the prevalence. In addition, truncal or central obesity is associated with an abnormal adipocyte activity, leading to secretion of various peptides that contribute to metabolic abnormalities. Whether obesity is a comorbid condition associated with OSA or is actually the primary problem and OSA is a consequence of the obesity is still in question. Epidemiologic studies so far have not found complete answers to the questions raised regarding the relationship between obesity and OSA.
Definition of Abnormal Breathing Events
An apnea is defined as complete or near cessation of airflow for a minimum of 10 seconds with or without associated oxygen desaturation or sleep fragmentation (arousal defined by electroencephalography [EEG]),16 although they are both usually present. The definition of a hypopnea is still under debate. Thermistors that monitor change in air temperature are considered less sensitive for detecting airflow compared to nasal cannula pressure transducers that measure change in pressure induced by airflow. The nasal cannula pressure transducer is the recommended equipment used to monitor airflow because it is considered a more accurate indicator of change in the nasal flow curve. The nasal cannula pressure transducer permits inference regarding airway resistance through presentation of the inspiratory airflow wave contour.17,18 Normally, in the absence of upper airway narrowing or obstruction, the inspiratory airflow waveform is characterized by a rounded peak contour. In the setting of increased airway resistance, which is usually associated with increased inspiratory effort, there is a plateau or flattening of the peak or an abrupt decrease immediately following an initial peak in the wave contour.
The American Academy of Sleep Medicine (AASM) has recommended that a hypopnea be defined by a 30% or greater reduction in the amplitude of the nasal wave contour from baseline and associated with at least 4% oxygen desaturation from the pre-event oxygen saturation, or a 50% or more decrease in the amplitude of the airflow wave contour from baseline in association with at least 3% oxygen desaturation from the pre-event oxygen saturation, or an EEG arousal. When reading the literature, it is important to recognize that even minor differences in event definitions can have a substantial impact on the study data. For example, using the criteria proposed by AASM in 1999 and 2007, a study comparing the scoring criteria of hypopneas with at least 30% decrease in flow amplitude and EEG arousal, or with at least 3% oxygen desaturation in 35 normal-weight 25- to 48-year-old subjects with suspected abnormal breathing during sleep reported that AHI scores could change from 26.9 ± 7.3 to 6.4 ± 3.1 events per hour of sleep when using EEG arousal versus oxygen desaturation criteria, respectively.19
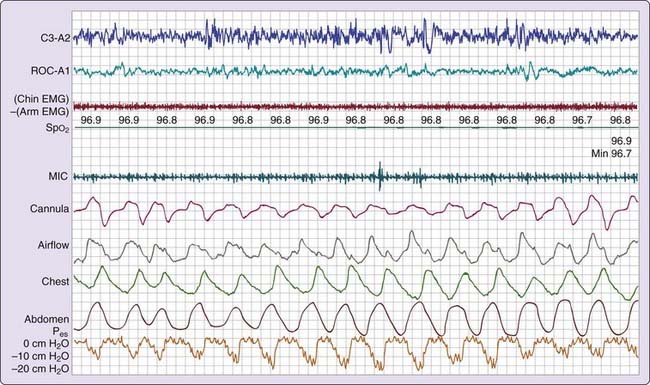
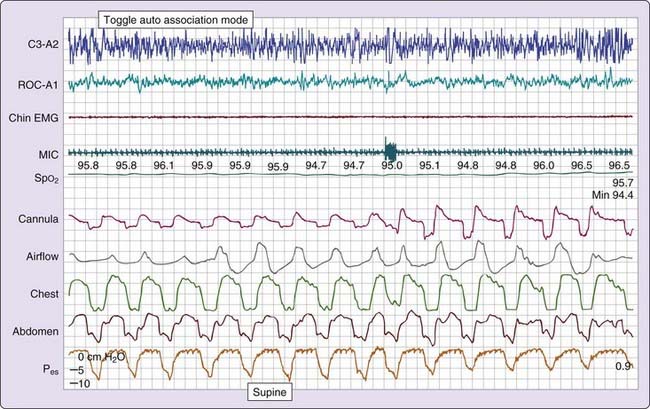
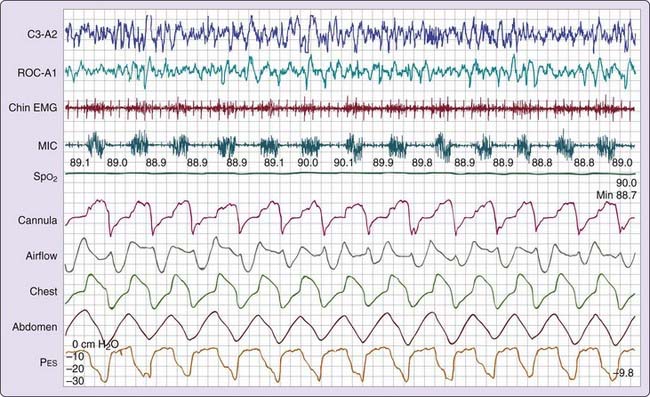
Certain persons with daytime symptoms similar to those endorsed by OSA patients display a pattern indicating increased inspiratory effort, which, in the absence of the currently defined apneas and hypopneas, still leads to sleep disruption (e.g., arousal or awakening).20 It is believed that the sleep disruptions result in significant daytime symptoms such as excessive fatigue or sleepiness. The increase in inspiratory effort can be detected by esophageal manometry (gold standard) or inferred from the signal provided by the nasal cannula pressure transducer17,18 Esophageal pressure (Pes) provides a reflection of intrathoracic pressure fluctuations associated with breathing efforts. The greater the inspiratory effort, the greater the oscillations in Pes. Normally, the most negative Pes value generated during inspiration (measured from the end of expiration to the most negative pressure during inspiration) is 2 to 3 cm H2O in a small woman and 8 to 9 cm H2O in a healthy large man.21
Three patterns of increased inspiratory effort are recognized by Pes measurements. First, Pes crescendo22 reflects progressively more negative breath-by-breath swings in esophageal pressure terminating either with an alpha wave or a mixture of alpha and beta wave EEG arousal, or with a burst of delta wave activation.23 This entity that is formed by the alpha wave or alpha and beta wave bursts is referred to as a respiratory effort–related arousal.1 Second, a pattern of sustained continuous inspiratory effort is a relatively stable but persistent increase in Pes over time (several epochs) terminated with EEG patterns similar to Pes crescendo. Third, the pattern of Pes reversal is an abrupt drop in the esophageal pressure after a sequence of increased inspiratory efforts, independent of the EEG pattern seen (Figs. 105-1, 105-2, and 105-3).21
Clinical Signs and Symptoms
The bed partner of an OSA patient often witnesses snoring (see Chapter 102), nocturnal snorting and gasping, and apneas.24 Nocturnal (sleep-related) symptoms and signs tend to be more specific for OSA than those expressed during diurnal wakefulness such as excessive daytime sleepiness, which is usually the result of abnormal sleep regardless of the cause. The OSA patient might report symptoms of tiredness, fatigue, or drowsiness rather than overt daytime sleepiness.
Nighttime Signs and Symptoms
Almost all OSA patients and many UARS patients snore. Snoring can be extremely loud and disruptive. A characteristic pattern in OSA is loud snoring or brief gasps alternating with intervals of silence that terminate with a loud snort or gasp reflecting resumption of breathing.24,25 The complaint of snoring often precedes the complaint of daytime sleepiness, and the intensity increases with weight gain and bedtime alcohol intake. Snoring can be a factor in marital discord; in one study, 46% of patients slept in separate bedrooms from their partners.24 Even though snoring is an important clue, the absence of its report does not exclude the presence of OSA.
Pregnancy is associated with an increase in snoring due to the upward displacement of the diaphragm (which reduces lung volume and its stabilizing effect on the upper airway) and nasopharyngeal edema (see Chapter 101). Although up to 30% of pregnant women snore, overt OSA is uncommon. Chronic snoring is seen in 5 to 8% of all pregnant women (including those with preeclampsia). However, the relationship between OSA and preeclampsia is still unclear at this time. To date, despite preliminary data associating the development of early hypertension in pregnant patients with SDB, no direct linkage has been demonstrated.26–30
Observed apneic episodes are reported by about 75% of bed partners.31 Persistent chest movements can usually be observed during periods of obstructive apneas. Observing apneic periods can cause the bed partner considerable anxiety, and many respond by shaking the patient for fear that breathing might not resume. Apneic episodes are usually terminated by gasps, chokes, snorts, vocalizations, or brief awakenings. Bed partners report a sudden cessation of snoring followed by a loud snort and resumed snoring. Although some patients awaken with a sensation of having stopped breathing, most are unaware of the apneas. A sensation of choking or dyspnea that interrupts sleep is reported by 18% to 31% of patients.24,32,33 On the other hand, particularly in the elderly, there is awareness of frequent unexplained awakenings, and this group tends to complain of insomnia and unrefreshing sleep.
About half of OSA patients report restless sleep (tossing and turning) and diaphoresis, usually localized to the neck and upper chest area (Video 105-1![]() ).32,33 These symptoms are probably related to increased breathing effort during periods of upper airway obstruction. Bed partners readily attest to excessive body movements because movements can at times be violent. Bed covers may be noted to be disheveled the morning. During episodes of upper airway obstruction, progressively more vigorous inspiratory efforts lead to more negative intrathoracic pressure swings, with an increase in venous return to the right ventricle. Large negative intrathoracic pressure swings can also adversely affect left ventricular function. These circumstances can increase pulmonary capillary wedge pressure and contribute to the sensation of dyspnea.34 Because nocturnal dyspnea can also occur in patients with congestive heart failure (paroxysmal nocturnal dyspnea) in the absence of SDB, it is important to inquire about other symptoms in order to distinguish heart failure from OSA.35 However, it is not unusual for the two disorders to coexist. In our experience, nocturnal dyspnea due to OSA usually resolves quickly upon awakening, whereas the paroxysmal nocturnal dyspnea that is characteristic of congestive heart failure takes much longer to resolve.
).32,33 These symptoms are probably related to increased breathing effort during periods of upper airway obstruction. Bed partners readily attest to excessive body movements because movements can at times be violent. Bed covers may be noted to be disheveled the morning. During episodes of upper airway obstruction, progressively more vigorous inspiratory efforts lead to more negative intrathoracic pressure swings, with an increase in venous return to the right ventricle. Large negative intrathoracic pressure swings can also adversely affect left ventricular function. These circumstances can increase pulmonary capillary wedge pressure and contribute to the sensation of dyspnea.34 Because nocturnal dyspnea can also occur in patients with congestive heart failure (paroxysmal nocturnal dyspnea) in the absence of SDB, it is important to inquire about other symptoms in order to distinguish heart failure from OSA.35 However, it is not unusual for the two disorders to coexist. In our experience, nocturnal dyspnea due to OSA usually resolves quickly upon awakening, whereas the paroxysmal nocturnal dyspnea that is characteristic of congestive heart failure takes much longer to resolve.
Complaints that suggest nocturnal gastroesophageal reflux and to a lesser degree diurnal gastroesophageal reflux (e.g., heartburn) are often expressed by OSA patients. Vigorous respiratory efforts by the patient during periods of apnea and hypopnea result in greater negative intrathoracic pressure swings during inspiratory efforts while there is relatively increased intraabdominal pressure during expiration (measurements of abdominal muscle activity in have shown that there is an active participation of abdominal muscles during expiration in many OSA subjects). Reflux results when an increased gradient between intra-abdominal and intrathoracic pressures favors the movement of gastric contents cephalad into the esophagus. In our experience and that of others, reflux can lead to laryngospasm.36 Untreated reflux can make patients more susceptible to nocturnal aerophagy when treated with nasal continuous positive airway pressure (CPAP).37
Nocturia is relatively common in patients with OSA: 28% of patients report four to seven nightly trips to the bathroom.38 OSA can be an independent cause of frequent urination during sleep in elderly men, and patients might initially consult urologists and attribute their disrupted sleep to repeated nocturia.39 CPAP can significantly decrease the frequency of nocturia and improve quality of life.40 Rarely, an adult patient complains of enuresis. Increased intraabdominal pressure, confusion associated with arousals, and increased secretion of atrial natriuretic peptide40 are proposed contributors to nocturia and enuresis.
About 74% of patients with OSA report dry mouth and the desire to drink water either during the night or in the morning.24 On the other hand, about 36% of patients with OSA complain of drooling. These symptoms are useful indicators of evidence for SDB, particularly in premenopausal women, who often have more limited and atypical clinical presentations.41 Dry mouth and drooling are most likely due to mouth breathing during sleep, which is commonly seen in patients with OSA. OSA is also commonly associated with nocturnal bruxism.42
Daytime Signs and Symptoms
Daytime sleepiness or fatigue is the most common complaint in patients with OSA.24,31,32 The manifestations of sleepiness can have subtle consequences (midafternoon drowsiness during a group meeting or an occasional nap), severe consequences (falling asleep while eating or talking), or catastrophic consequences (falling asleep while driving) (see Video 104-1![]() ). In general, it is not normal to feel sleepy immediately after a meal or while watching television. Such drowsiness usually indicates some degree of sleep deprivation or fragmentation, and OSA may be the underlying factor. It is essential to inquire about sleepiness while operating a machine or motor vehicle because of the increased risk of accidents7–9,24,31–33,43–47 and concern regarding bystanders’ safety.
). In general, it is not normal to feel sleepy immediately after a meal or while watching television. Such drowsiness usually indicates some degree of sleep deprivation or fragmentation, and OSA may be the underlying factor. It is essential to inquire about sleepiness while operating a machine or motor vehicle because of the increased risk of accidents7–9,24,31–33,43–47 and concern regarding bystanders’ safety.
In the sleep laboratory, pathologically short sleep latency, objectively assessed by the maintenance of wakefulness test, is associated with impaired driving in a simulator environment.45 Driving tests and simulations have shown that OSA patients might not be aware of their degree of impairment while driving. Clinical evidence for impaired driving ranges from subtle to dramatic; some drivers recall an occasional honk from the car behind alerting them to the changing of traffic light; others report routinely rolling down the window or drinking coffee to stay awake,45 and others report falling asleep while in motion, resulting in riding on the berm or a crash. Some pathologically sleepy patients deny any problems with driving due to fear of restriction on their license. Studies have shown that there is a correlation between the Maintenance of Wakefulness Test (MWT) and the risk of impaired driving in OSA patients.46,47 However, one of the same studies showed that OSA patients with mildly abnormal results in the MWT had no driving impairment.47
Causes of sleepiness in OSA patients are multifactorial. Nocturnal disruption may be secondary to repetitive abnormal breathing events leading to arousals or complete awakenings in the middle of night or to early morning awakenings, with subsequent complaints of insomnia and reduced total sleep time. Mood disorders that are associated with disturbed nocturnal sleep can coexist with OSA. OSA and obesity often coexist, and whereas the former is associated with increased sleepiness, obesity per se has also been associated with sleepiness. In this regard, obesity may be responsible for the residual sleepiness that has been reported in obese patients who are otherwise appropriately treated for OSA with nasal CPAP.48 In a large survey of clinic patients, Stoohs and colleagues49 found that UARS patients had worse morning performance on the psychomotor vigilance task (PVT) compared to OSA patients who had apneic events that were longer in duration than the flow-limited events leading to sleep disturbance in UARS patients.
Obstructive events may terminate with only increased delta on EEG rather than an EEG arousal.23 A study examining noise-induced brainstem activation versus EEG arousal during one nocturnal sleep period during which there were about 200 stimulations showed that the MSLT and PVT were impaired only when EEG arousals were present and not when brainstem activation was induced.50 Studies of sleep restriction lasting 1 week or longer have shown that responses were very different across subjects.51 It is postulated that genetic differences might explain why some subjects are more susceptible to nocturnal sleep disruption and restriction than others.
While sleepiness might not be directly perceived by patients, they may complain of “tiredness” or “fatigue.” When asked to rest in a seated position with muscles relaxed to see if the feeling of fatigue could be improved, subjects denied improvement and differentiated between exercise-related fatigue and an overall feeling of fatigue. Subjects also complained that they experienced “lapses” while driving or performing monotonous tasks and reported lack of memory intermittently for a limited time period.45,47 Moreover, rather than, or in addition to sleepiness, patients may report cognitive dysfunction such as concentration, attention, memory, or judgment difficulties affecting their job performance (see Chapter 104). These complaints were most common in premenopausal women with a low AHI (mean, 13 events per hour of sleep) or UARS patients.41 In men performing manual work requiring attention and dexterity, unexplained periods of clumsiness were mentioned in association with concentration difficulties. These issues may be sufficiently substantial that job performance or even the ability to maintain employment is adversely affected.
Most patients seem to respond favorably to treatment with nasal CPAP, with return to normal performance. However, some patients still complain of symptoms despite appropriate nasal CPAP use, and these patients need further investigation into the cause of their cognitive dysfunction. As is the case with the individual propensity to daytime sleepiness, it is not clear why some patients are more susceptible to cognitive dysfunction. Genetic influences might play a role; as an example, the APOE4 gene that is associated with neurodegenerative disorders has been clearly identified in OSA patients (see Chapter 103).52 However, the APOe-4 gene may not be directly linked to OSA specifically but may be a gene that favors neuro-degenerative disease secondary to several different types of central nervous system insults. It is possible that specific genetic susceptibilities favor persistent neurocognitive dysfunction when exposed to an environmental condition such as OSA. Premenopausal women with a low AHI or UARS might have symptoms similar to attention-deficit/hyperactivity disorder (ADHD), and may have received a mistaken diagnosis of adult ADHD.41
Personality changes including aggressiveness, irritability, anxiety, or depression may be observed. Treatment with continuous positive airway pressure (CPAP) has been shown to improve symptoms such as depression and fatigue.53,54 In our experience, a third of the patients report decreased libido or impotence that tends to improve with treatment. In addition, about 75% of OSAS patients with erectile dysfunction who have been treated with nasal CPAP report disappearance of difficulties with erection or ejaculation, resulting in significantly improved quality of life.55
Morning or nocturnal headaches are reported in about half of the patients and are often described as dull and generalized.56 Headaches usually last 1 to 2 hours and can prompt the ingestion of analgesics. A study from a clinic specializing in headaches found OSA to be the main cause of nocturnal or morning headaches.56 None of these patients had been previously investigated for OSA. Nocturnal headaches were controlled by OSA treatment.57
It is unlikely that all of these signs and symptoms will be reported by one person. There is a notable lack of specificity for most of these symptoms, and they overlap with other disorders such as depression or hypothyroidism.58 In many patients with depression, the presence of daytime sleepiness should prompt a sleep study to rule out coexisting OSA. With respect to thyroid abnormalities, one study detected hypothyroidism in 3 of 103 patients (2.9%) with OSA versus 1 of 135 control subjects (0.7%),59 and this difference was not significant. The authors concluded that routine thyroid function testing is not indicated in the absence of signs or symptoms of hypothyroidism, although an exception can be made for high-risk groups such as women older than 60 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree