18 Dementia
Mild Cognitive Impairment, Alzheimer Disease, Lewy Body Dementia, Frontotemporal Lobar Dementia, Vascular Dementia
Mild Cognitive Impairment
The utility of these tests in detecting MCI is less reliable. Indeed, most patients with MCI score within the normal range on the MMSE. Interview-based dementia assessments, such as the Clinical Dementia Rating scale (CDR), provide a more sensitive means for reliable detection of MCI but may require considerably more time to administer. Another brief screening tool, the Montreal Cognitive Assessment Test (www.mocatest.org), may provide greater sensitivity in detecting MCI. The definitive diagnosis of MCI requires formal neuropsychological assessment. However, neuropsychological test batteries take several hours to administer and interpret. Therefore, they are not practical as screening tools. In the hands of an experienced neuropsychologist, formal neuropsychological tests provide the most sensitive means of detecting cognitive impairment. They may also provide greater specificity in identifying the underlying cause, although there may be significant variability among neuropsychologists’ interpretations.
Alzheimer Disease
Pathogenesis
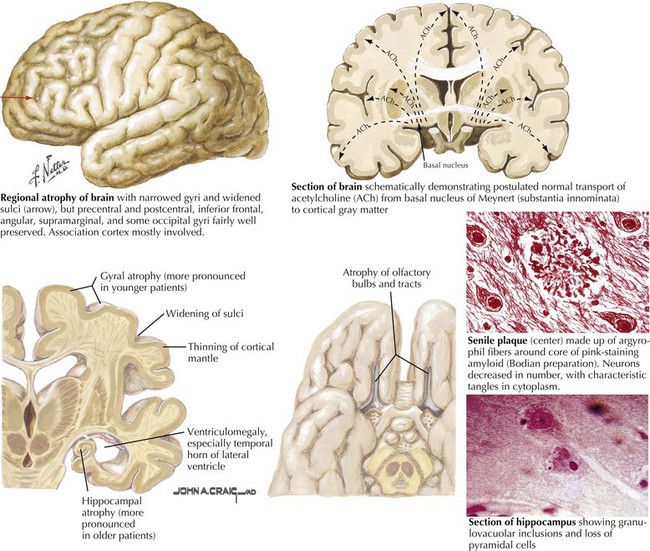
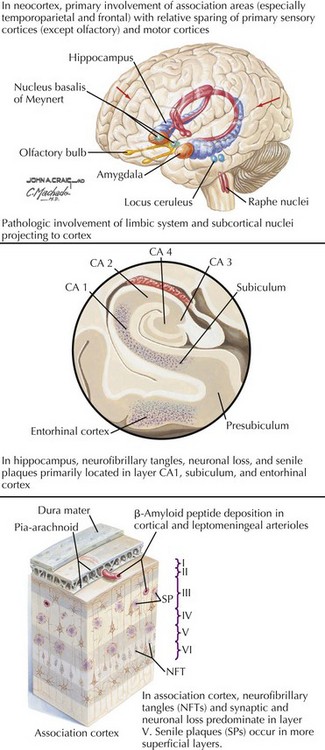
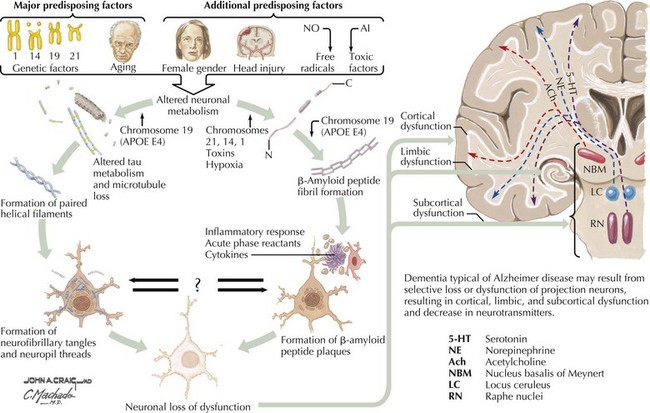
There is pronounced gross cerebral atrophy clearly evident on both imaging studies and post mortem. Typically, the dementia of AD preferentially affects the frontal, temporal, and parietal cortex. This is particularly evident in the temporoparietal and frontal association areas as well as the olfactory cortex. In contrast, other primary sensory cortical areas are unaffected. Additionally, the limbic system as well as subcortical nuclei as well as the nucleus basalis of Meynert are preferentially affected. Microscopically, there is clear loss of both neurons and neuropil. The classic findings include senile plaques and neurofibrillary tangles (Figs. 18-1 and 18-2). The white matter sometimes demonstrates a secondary demyelination.
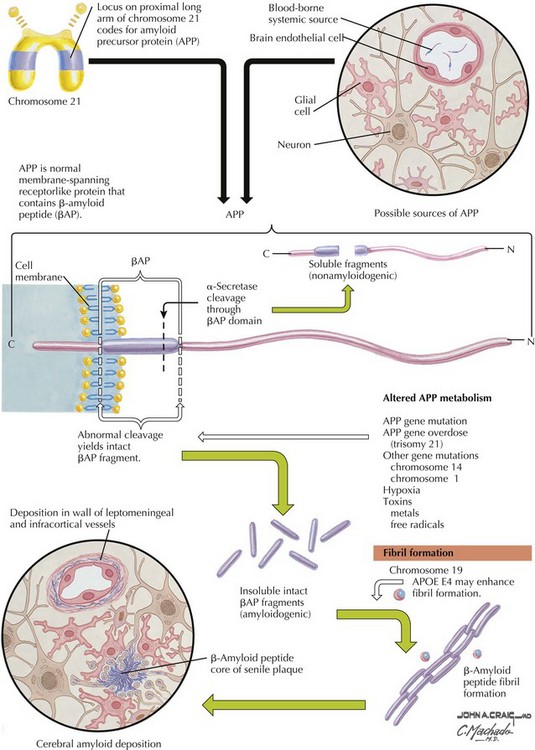
β-Amyloid
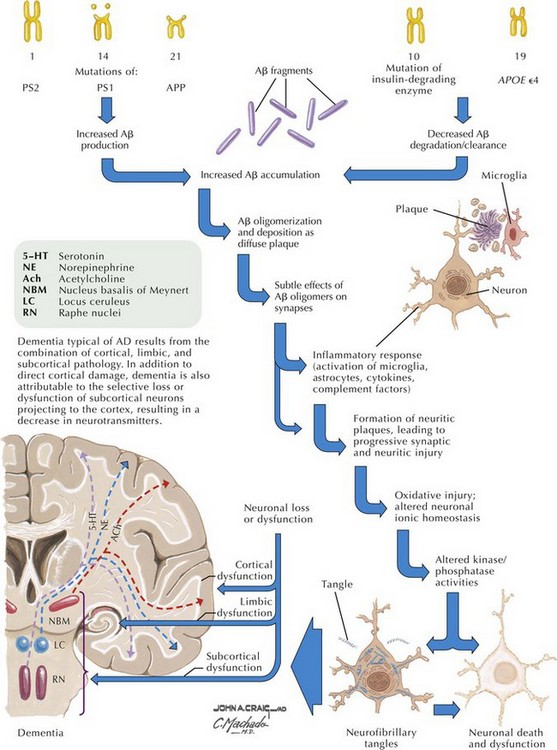
β-Amyloid is a short fragment of the APP, typically 40–42 amino acids in length, which accumulates outside the cell during APP processing (Figs. 18-3 and 18-4). The tertiary structure of the 42–amino acid fragment is a β-pleated sheet that renders it insoluble. Consequently, it accumulates slowly, over many years, in the extracellular space and within synapses. In vitro studies confirm that β-amyloid is toxic to surrounding synapses and neurons, causing synaptic membrane destruction and eventual cell death. Transgenic mouse models show a clear association between accumulation of β-amyloid fragments, formation of amyloid plaques, and development of cognitive impairment.
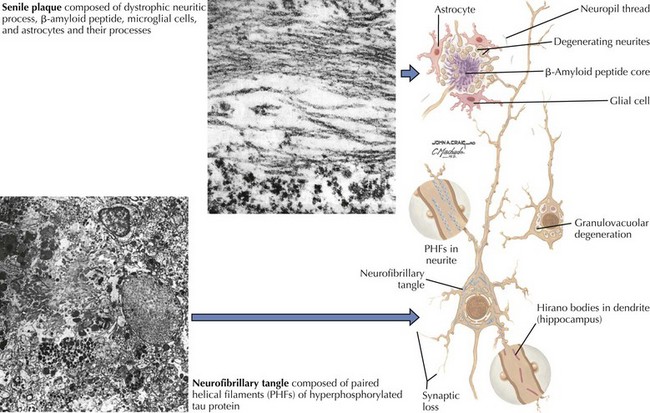
In vivo, β-amyloid fragments coalesce to form “diffuse” or immature plaques, best seen with silver-staining techniques. Diffuse plaques, however, are not sufficient to produce dementia; many nondemented elderly patients have substantial depositions of diffuse plaques throughout the cortex, a condition termed pathologic aging. It is when these plaques mature into “senile” or neuritic plaques that dementia becomes more likely (Fig. 18-5, top). Senile plaques consist of other substances in addition to β-amyloid, including synaptic proteins, inflammatory proteins, neuritic threads, activated glial cells, and other components. Unlike diffuse plaques, senile plaques are composed of a central core of β-amyloid surrounded by a myriad of proteins and cellular debris. Senile plaques are distributed diffusely in the cortex, typically starting in the hippocampus and the basal forebrain. Senile plaque formation correlates with increasing loss of synapses, which correlates with the earliest clinical sign, namely, short-term memory loss. The anatomic pattern of progression gradually spreads to neocortical and subcortical gray matter of the temporal, parietal, frontal, and, eventually, occipital cortex. Subcortical nuclei become involved relatively late in the process.
Neurofibrillary Tangles
The second pathologic hallmark of AD is the neurofibrillary tangle (Fig. 18-5, bottom). These lesions develop and conform to an anatomic pattern that correlates with the clinical syndrome; the number and distribution of tangles are directly related to the severity and clinical features of the dementia. Neurofibrillary tangles form intracellularly, consisting of a microtubule-associated protein, tau, which has a vital role in the maintenance of neuronal cytoskeleton structure and function. Tau is hyperphosphorylated in AD, causing it to dissociate from the cytoskeleton and accumulate, forming a paired helical filament protein structure. The cytoskeleton is compromised structurally and functionally, disrupting normal cell function. The most commonly used pathologic criteria for definitive AD diagnosis at autopsy require the presence of senile plaques and neurofibrillary tangles. Other lesions, such as Hirano bodies, are also seen in AD but have little diagnostic specificity.
Neurotransmitters
In addition to neuronal and synaptic loss, there is a gradual loss of various neurotransmitters. Acetylcholine synthesis is the earliest and most prominently affected. Most acetylcholinergic neurons arise within the nucleus basalis of Meynert in the basal forebrain (see Fig. 18-2). This nucleus is affected relatively early in the process; acetylcholine levels within the brain and spinal fluid of patients with AD quickly decline with disease progression. This observation supported the cholinergic hypothesis—that acetylcholine depletion results in the cognitive decline observed in patients with AD—eventually leading to the first symptomatic treatment of AD.
Risk Factors
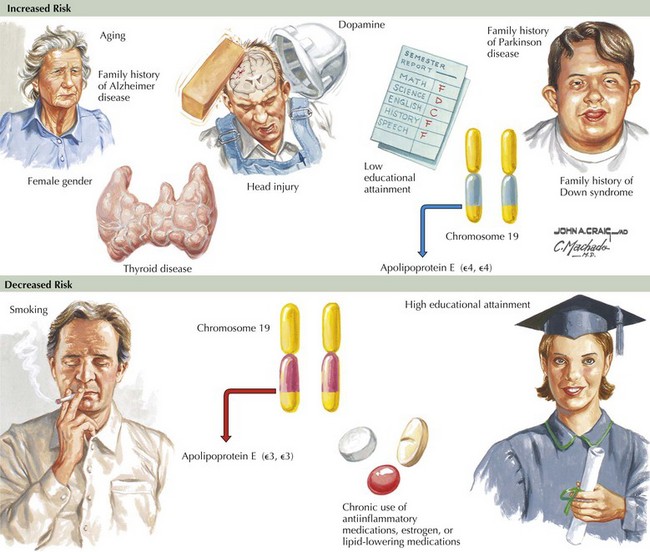
Epidemiologic studies identify several potential risk factors for AD. The most consistent risk factors include advanced age, family history (especially in first-degree relatives), and ApoE genotype. Other risk factors include hypertension, stroke, and fasting homocysteine levels (Fig. 18-6). Because vascular risk factors are modifiable, they may affect risk reduction and treatment for patients with AD and those at risk for development of AD.
Clinical Presentation
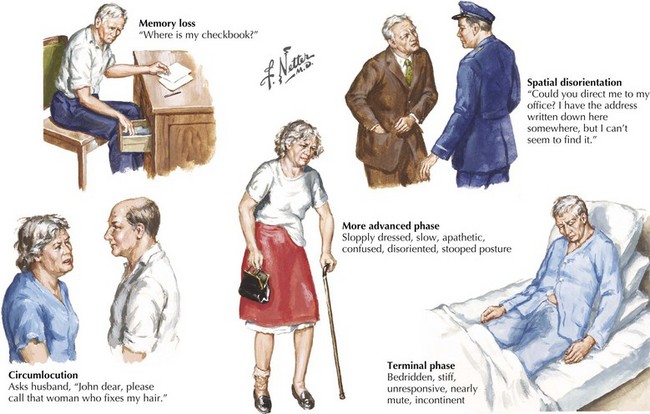
The early signs of AD may be subtle (Fig. 18-8). In the initial stages of AD, memory losses can be clinically distinguished from normal aging, although formal memory testing is often required to confirm suspicion of early dementia. The early signs of Alzheimer begin insidiously, progress slowly, and are often covered up by patients. Detection may be challenging even for close family members. The physician may observe changes in patient’s pattern of behavior, such as missing appointments or poor compliance with medications. It is important to discuss such issues openly with family members given the patient often cannot recall examples of memory problems. Indeed, it is common for patients to have limited insight into their deficit, and for family members to initiate an evaluation for memory loss. In these early stages, patients maintain their social graces. It is not uncommon during mental status testing to discover the significant cognitive problems concealed by a patient’s friendly and sociable affect. “Very pleasant” patients sometimes fool even seasoned geriatricians. The Alzheimer’s Association lists 10 key warning signs of AD.
Differential Diagnosis
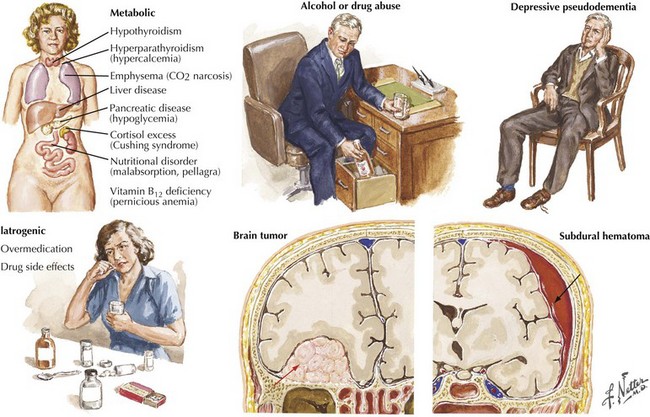
The absence of motor deficits early in AD differentiates it from most other dementias. Other dementias lacking motor signs include amnestic syndrome (Korsakoff encephalopathy), Pick disease, vascular dementia, and HIV dementia complex. Depression can also produce dementia-like symptoms without motor deficits. Poor concentration and short-term memory impairment result from lack of effort, disinterest, or distractibility. “Pseudo dementia” due to depression is usually not progressive, and functional loss is often disproportionately severe relative to cognitive impairment (Fig. 18-9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree