33 Parkinson Disease
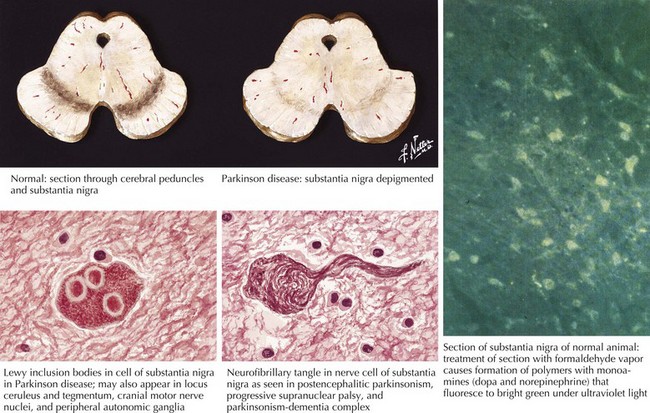
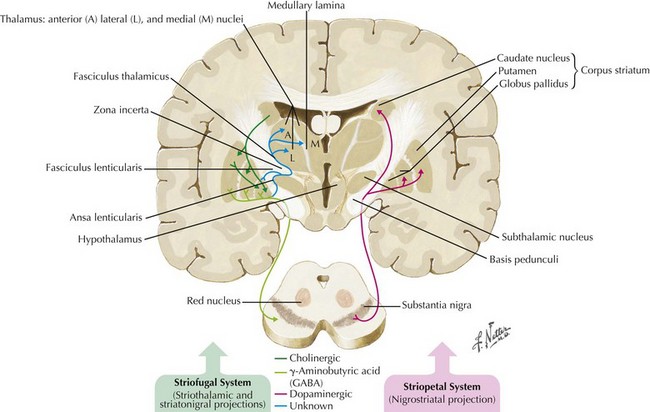
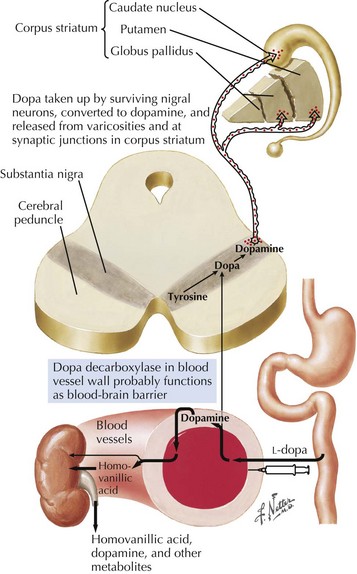
In 1817, James Parkinson made the seminal observations on this disorder defining a specific neurodegenerative illness characterized by bradykinesia, resting tremor, cogwheel rigidity, and postural reflex impairment. Parkinson disease (PD) has a relatively stereotyped clinical presentation that now bears the name of this early 19th-century physician. PD is one of the most common neurologic disorders worldwide. It affects at least 1,500,000 persons within the United States. Its incidence typically peaks in the sixth decade; however, one can see classic clinical cases with onset as early as the fourth decade. On occasion, medication-induced Parkinson disease may also occur in early middle-aged individuals. In contrast, PD sometimes presents well into the late eighth or even the ninth decade. Usually the patient’s clinical status progresses from a relatively modest limitation at diagnosis to an ever-increasing disability over 10 to 20 years in many but not all patients. The primary neuropathologic features are loss of pigmented dopaminergic neurons mainly in the substantia nigra (SN) and the presence of Lewy bodies—eosinophilic, cytoplasmic inclusions found within the pigmented neurons (Fig. 33-1). These neurons’ primary projection is to the striatum, for example, the putamen and caudate. Dopamine is released primarily from these striatal cells. From here dopamine neurotransmission sequentially is directed through the globus pallidus, the subthalamic nucleus, to the thalamus per se, and then to the primary motor cortex (Figs. 33-2 and 33-3).
Etiology
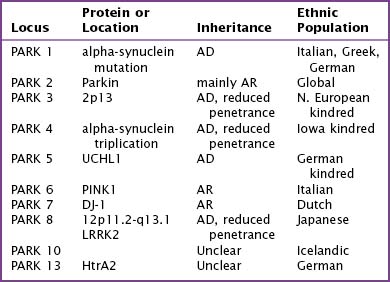
Fifteen percent of Parkinson patients have a family history of PD; a small percentage of these individuals have at least three affected generations. It is unknown whether the clinical picture results from a defective gene per se, a shared environmental insult, or both. Currently there are several causative genes identified that are specific to young-onset PD. Although these well-identified PD genes are pathogenic in only a very small minority of individuals, their biochemical signatures are providing extraordinary insight into the molecular pathology of this disease. The currently identified genes are listed in Table 33-1.
Genes for Parkinson Disease
Alpha-Synuclein
The discovery that mutant AS could cause PD quickly led to the discovery that AS also served as the principal component of Lewy bodies. These are proteinaceous cytoplasmic inclusions specifically found in the brains of PD patients; however, even today, the exact role of Lewy bodies is still unknown (see Fig. 33-1). The confirmation that triplication of the AS gene and the subsequent elevated protein levels (in the CSF) could cause PD has greatly strengthened the hypothesis that accumulation of protein is a fundamental event in the pathogenesis of PD.
Pathology/Pathophysiology
The pathologic sites responsible for the parkinsonian disorders reside in a group of brain gray matter structures known as the extrapyramidal system or basal ganglia (Fig. 33-2). These include striatum (caudate nucleus and putamen), globus pallidus interna and externa, subthalamic nucleus, substantia nigra pars reticulate and pars compacta, and the ventral nuclei of the thalamus.
Degeneration of the substantia nigra (SN) pars compacta is the pathologic hallmark of PD (Figs. 33-2 and 33-4). Neurons within the SN per se synthesize the neurotransmitter dopamine. These cells contain a dark pigment called neuromelanin. Parkinson symptoms develop when approximately 60% of these cells die. Concomitantly, direct inspection of the SN in PD demonstrates an abnormal pallor when compared with that characteristically seen with the normal hyperpigmented melanin-containing cells.
Clinical Presentation
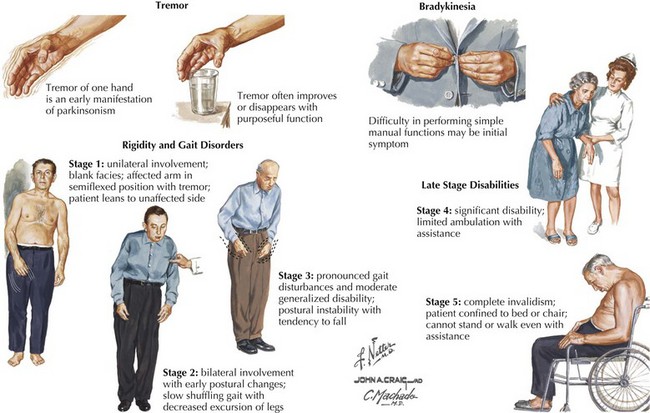
The four primary signs of PD are bradykinesia, tremor, rigidity, and gait disturbance (Fig. 33-5). The primary criteria for a diagnosis of PD require that the patient’s neurologic examination demonstrate at least two of these four features. There are certain additional features very suggestive of idiopathic PD. These include an asymmetric or unilateral onset and a clear response to levodopa treatment. Importantly from the point of differential diagnosis, neither of these features occurs in some of the atypical parkinsonism syndromes.
Typically PD progresses in stages (Fig. 33-5). There are two commonly utilized rating scales to measure the degree of disability that these patients manifest: (1) UPDRS (Unified Parkinson Disease Rating Scale) and (2) Hoehn and Yahr (H&Y) scale (Box 33-1).