CHAPTER 70 DEMENTIA WITH LEWY BODIES
HISTORICAL PERSPECTIVE
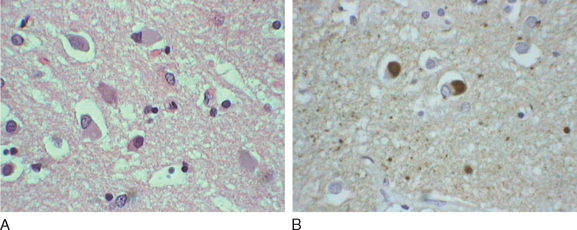
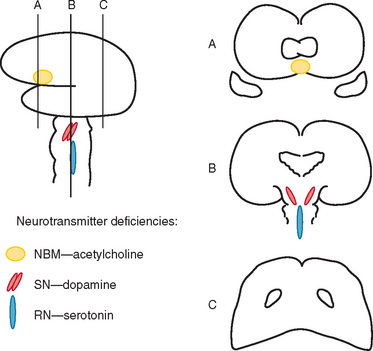
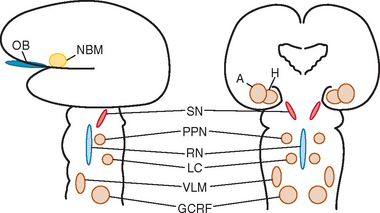
Okazaki and colleagues first described Lewy bodies in the cerebral cortex in patients with dementia in 1961.1 Few reports on Lewy body cortical pathology were published over the subsequent several years, perhaps partly because of the challenge of identifying Lewy bodies on standard hematoxylin- and eosin-stained cortical regions. In the 1980s, ubiquitin immunocytochemistry allowed easier recognition of Lewy bodies, but structures other than Lewy bodies are stained with ubiquitin.2,3 α-Synuclein immunocytochemistry was developed in the late 1990s, permitting easy identification of Lewy bodies and Lewy neurites (Fig. 70-1).4,5 Investigators from Japan, Europe, and the United States have pioneered much of the clinical and pathological characterization of Lewy body dementia/Lewy body disease since the mid-1980s.2–27 It is now clear that multiple neurotransmitter systems and structures in the brain are dysfunctional in dementia with Lewy bodies (DLB) (Figs. 70-2 and 70-3).
TERMINOLOGY
The terminology in the clinical and pathological characterization of Lewy body disease (LBD) has been confusing. Terms have included Lewy body disease, diffuse Lewy body disease, cortical Lewy body disease, Lewy body dementia, the Lewy body variant of Alzheimer’s disease, and senile dementia of the Lewy type. In 1995, the Consortium on Dementia with Lewy Bodies developed the consensus criteria for the clinical and neuropathological diagnoses. The report, published in 1996, suggested that the syndrome be termed dementia with Lewy bodies and that the neuropathological disorder be termed Lewy body disease.6 Therefore, most experts restrict use of the term dementia with Lewy bodies to the clinical syndrome; when characterizing autopsied material, the term Lewy body disease is used instead.
EPIDEMIOLOGY
The frequency of a DLB, based primarily on cases in hospital- and referral-based samples, has been approximately 15% to 25% of cases of irreversible dementia.6 More recently, in an autopsy-based study of dementia subjects in Olmsted County, Minnesota, the frequency of LBD was 10%.28 A population-based study in Finland suggested that among persons aged 75 years of age and older, the prevalences for dementia and DLB specifically were 22% and 5%, respectively.29 The few published data on frequency or prevalence suggest that DLB and LBD probably account for fewer than 25% of dementia cases and are closer to 5% to 15%. Prevalence may vary among populations, but data are inadequate for concluding whether there are significant differences on the basis of race or ancestral origin. No studies on incidence have been published to date. There does appear to be a male preponderance in DLB.6–8
CLINICAL FEATURES
A wide spectrum of symptoms and signs can occur in DLB (Table 70-1).6–8,24,27,30–34 Most symptoms can be categorized into one of five categories: cognitive impairment, neuropsychiatric features, motor dysfunction, sleep disorders, and autonomic dysfunction.
TABLE 70-1 Clinical Features Often Present in Dementia with Lewy Bodies
REM, rapid eye movement.
From Boeve B: Dementia with Lewy bodies. In Petersen R, ed: Continuum. Minneapolis: American Academy of Neurology, 2004, pp 81-112.
Cognitive Impairment
Executive and visuospatial functioning are the domains most consistently impaired in DLB.35 Symptoms of executive dysfunction include changes in problem solving, performance of sequential tasks, multitasking, and complex decision making. Difficulties with navigating in familial surroundings and problems sitting on a sofa or lying in the correct orientation on a bed are commonly voiced symptoms of visuospatial dysfunction. Memory impairment can vary from slight to very severe.35 Verbal blocking, in which a person tends to lose the train of thought in the middle of a sentence, is very common; this phenomenon can be mistaken for dysarthria or aphasia. Apathy and bradyphrenia are also common. Misidentification errors involving people can occur and are particularly upsetting when patients fail to recognize their own spouses or children. Reflections in mirrors may be mistaken for other individuals, and patients may speak to or argue with the perceived person. Most clinicians have regarded these cognitive symptoms as reflecting dysfunction of the frontosubcortical and parieto-occipital neural networks, as well as cholinergic depletion.
Fluctuations—periods of time when cognition and arousal are near normal and other periods of more marked confusion or decreased alertness—are considered a defining feature of DLB.6–8 The neural substrate responsible for fluctuations is not clear, but neurochemical alterations31 and sleep/wake dysregulation36 have been proposed.
Neuropsychiatric Features
Another defining feature of DLB is the presence of visual hallucinations.6,7,37 Often the hallucinations are first noted in the bedroom when the room is darkened at night; patients often visualize insects, animals, or people in the room, on the bed, or on the ceiling. These hallucinations are often vivid and fully formed, and patients often cannot be convinced that the stimuli are not truly present. In some, visual hallucinations can be frightening or can become the source of delusions (e.g., “That little girl has been stealing my money”). Visual illusions also are common and often coincide with the presence of visual hallucinations. Delusions are also frequent and typically have a paranoid quality, like the example just noted.37 Capgras’ syndrome—the belief that a relative or friend (usually spouse) has been replaced by an identical-appearing impostor—is also a feature of DLB.38 Depression occurs at some point in the illness in almost all patients with DLB, sometimes years before the onset of dementia.6,37 Anxiety is also common. Auditory, tactile, or olfactory hallucinations can also occur. Agitation and aggressive behavior are more variable; when present, they can be challenging to manage. Hypomania and overt bipolar disorder features can evolve, but they are atypical insofar as the onset usually occurs in patients in their 50s or 60s.
The underlying cause of hallucinations, delusions, and agitation probably reflects dopaminergic dysfunction, and serotonin dysfunction is probably involved in depression, anxiety, and bipolar-type features. Rapid eye movement (REM) sleep/wakefulness dysregulation has also been proposed as a mechanism underlying visual hallucinations in Parkinson’s disease and psychosis, in which the dream imagery of REM sleep may invade wakefulness.39 The same mechanism has been proposed to underlie hallucinations associated with DLB.24,33,34 The fact that psychostimulants can sometimes ameliorate hallucinations and delusions, which is similar to what occurs in narcolepsy, supports this hypothesis.24,33
Motor Dysfunction
Parkinsonism unrelated to dopamine antagonist exposure is another defining characteristic of DLB.6–8 Signs and symptoms include masklike facies, stooped posture, shuffling gait, difficulty with fine motor skills, sialorrhea, tremor, and bradykinesia.40 Tremor tends to be more symmetrical and related to postural/action, rather than the unilateral and predominantly at-rest tremor that is typical of Parkinson’s disease. Myoclonus can also occur, and when the clinical course is rapid, differentiation from Creutzfeldt-Jakob disease can be difficult. Many of these symptoms and signs result from reduced dopaminergic activity.
Sleep Disorders
REM sleep behavior disorder (RBD) is common in DLB (as well as in Parkinson’s disease with or without dementia and in multiple-system atrophy).22–27,33,34,41,42 Patients appear to be acting out their dreams by screaming, swearing, punching, and kicking (Table 70-2).23,24,34 The theme of the dream is remarkably consistent across patients, almost always involving chasing or attacking, and the patient is usually protecting himself or herself against aggressors rather than being the attacker. When the patient is awakened and able to recall the dream content, the description of the dream tends to match the behaviors that were exhibited. Injuries such as pulled hair, bruises, lacerations, and broken bones have been described in patients and their bed partners. RBD often begins years or even decades before any cognitive or motor symptoms develop, and therefore RBD may be the first sign of an evolving neurodegenerative disorder in many individuals. Excessive daytime somnolence, in which patients struggle to stay awake through the day, is also common.43,44 Other sleep disorders in DLB include insomnia, obstructive sleep apnea, central sleep apnea, restless legs syndrome, and periodic limb movement during sleep.42 In one polysomnographic series of DLB patients, at least one sleep disorder was present in almost every case.45 These sleep disorders are important to recognize because treatments exist for each one, and clinical improvement can be dramatic in some instances when all sleep disorders are effectively treated.

TABLE 70-2 Typical Clinical Features of REM Sleep Behavior Disorder
Rights were not granted to include this table in electronic media. Please refer to the printed book.
From Boeve B, Silber M, Ferman T, et al: REM sleep behavior disorder in Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. In Bedard M, Agid Y, Chouinard S, et al, eds: Mental and Behavioral Dysfunction in Movement Disorders. Totowa, NJ: Humana Press, 2003, pp 383-397.
Dysfunction in brainstem neuronal networks are believed to underlie RBD, particularly the pedunculopontine nucleus, locus ceruleus, and gigantocellularis reticular formation (see Fig. 70-3), although the specific networks have not been fully defined.23,24 α-Synuclein–positive pathology is often present in the lateral hypothalamus, which could explain the hypersomnolence and narcolepsy-like features. Central sleep apnea syndrome is also probably caused by brainstem dysfunction. The specificity of these sleep disorders to LBD is still being studied, but at least for RBD, it occurs almost exclusively in the synucleinopathies and rarely in other neurodegenerative disorders.23,24
Autonomic Dysfunction
Orthostatic hypotension, impotence, urinary incontinence, and constipation are common in DLB,46–48 although the frequency of each feature in DLB has not been systematically studied. Lewy bodies have been found in the intermediolateral column of the spinal cord and in the sympathetic nerves in the thoracic and abdominal structures, which reflects the rather widespread nature of Lewy body pathology in the central and peripheral nervous systems.47,49
DIAGNOSTIC CRITERIA
The criteria for the clinical diagnosis of DLB as per the Consortium on Dementia with Lewy Bodies (“McKeith criteria”), originally published in 1996,6 were refined in 1999.7 However, clinicopathological analyses have shown that the accuracy of the clinical criteria has varied widely among groups of investigators50–55; the specificity for the Consortium on Dementia with Lewy Bodies criteria is adequate, but sensitivity is relatively low. Further refinements of the criteria have been suggested through 2004 after several consensus meetings. The 2004 proposed criteria for the clinical diagnosis of DLB, the Third Report of the DLB Consortium55a, are shown in Table 70-3. Attempts continue to be made to operationally define criteria for fluctuations and better characterize the cognitive aspects, visual hallucinations, parkinsonism, sleep aspects, and autonomic aspects of DLB.

TABLE 70-3 Revised Criteria for the Clinical Diagnosis of Dementia with Lewy Bodies (DLB)
Rights were not granted to include this table in electronic media. Please refer to the printed book.
From McKeith IG, Dickson DW, Lowe J, et al: Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology 2005; 65:1863-1872.
For the neuropathological criteria, the First and Second Reports of the DLB Consortium suggested that ubiquitin immunohistochemistry be used and required counting Lewy bodies to characterize LBD as being predominantly brainstem, limbic, or neocortical. The recommendations from the Third Report of the DLB Consortium are to use α-synuclein immunohistochemistry and a semiquantitative grading of lesion density (Table 70-4) rather than the counting methods previously proposed, inasmuch as it was agreed that the pattern of regional involvement is more important than total Lewy body count. The grading involves categorizing Lewy body densities as mild, moderate, severe, and very severe and then assessing the regional pattern of Lewy-related pathology by grading it on a template similar to that used in the Consortium to Establish a Registry of Alzheimer’s Disease for neuritic plaques. Finally, the probability that the neuropathological findings are associated with a DLB clinical syndrome will be determined, taking accounts of both Alzheimer’s and Lewy body–type pathologies (Table 70-5). This schema requires validation studies but clearly is a solid refinement of the originally proposed neuropathological characterization of DLB and LBD.

Rights were not granted to include this table in electronic media. Please refer to the printed book.
From McKeith IG, Dickson DW, Lowe J, et al: Dementia with Lewy bodies: diagnosis and management: Third report of the DLB Consortium. Neurology 2005; 65:1863-1872.
DIAGNOSTIC EVALUATION
The primary diagnostic considerations in any patient with cognitive-behavioral changes include mild cognitive impairment, Alzheimer’s disease, DLB, frontotemporal dementia, and vascular dementia (reviewed in detail by Knopman et al56). The American Academy of Neurology practice parameter on the evaluation of individuals with dementia suggests that laboratory testing, neuropsychological testing, and structural neuroimaging be performed.57 Other diagnostic procedures that can aid in the evaluation of patients with possible DLB, particularly for differentiating DLB from Alzheimer’s disease, include electroencephalography, single photon emission computed tomography, positron emission tomography, polysomnography, autonomic studies, and smell testing. These tests are reviewed in more detail in the following sections.
Blood/Urine
The role of laboratory testing is most helpful in identifying treatable causes of cognitive impairment.57 No specific findings on laboratory testing of blood or urine that are characteristic of DLB have been identified as yet.
Cerebrospinal Fluid Analysis
On cerebrospinal fluid testing, low Aβ42 and normal tau levels58 have been reported in DLB, but this profile is also consistent with the diagnosis of Alzheimer’s disease. Hence, no specific findings on cerebrospinal fluid analysis that are diagnostic of DLB have been identified as yet.
Neuropsychological Testing
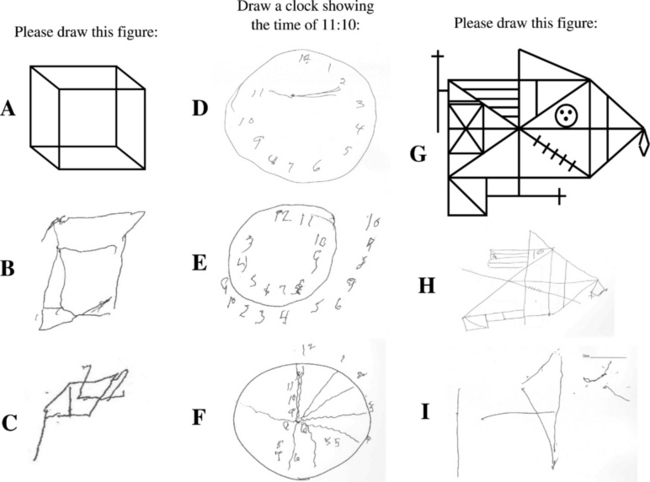
Neuropsychological testing typically reveals impairment on measures of attention/concentration and visuospatial functioning in DLB.13,35,59–61 Visuoconstructive abilities can be tested by having a patient draw a clock or copy the Necker cube, intersecting pentagons, and the Rey-Osterreith complex figure (Fig. 70-4). A similar pattern of deficits—impaired visual perceptual-organizational skills, constructional praxis, and verbal fluency—have been demonstrated in patients with dementia plus RBD.41 In a subsequent analysis in which the pattern of neuropsychological impairment was compared between one group of patients with RBD and dementia and another group of patients with autopsy-proved Alzheimer’s disease, a double dissociation was identified, in which the patients with RBD and dementia had worse impairment on measures of attention, visual perceptual-organizational skills, and letter fluency, whereas the patients with Alzheimer’s disease had had significantly worse performance on confrontation naming and verbal memory.25 The same pattern was then found in a group of patients who had dementia and RBD but did not have parkinsonism or visual hallucinations.26 These findings suggest that in the absence of visual hallucinations or parkinsonism, the presence of dementia and RBD may indicate underlying Lewy body disease. More recent neuropathological studies have corroborated this hypothesis.23,24 These studies strongly support the role of neuropsychological testing in the differential diagnosis in patients with dementia.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree