32
CHAPTER
![]()
Dietary Therapy
Candace Richardson and William B. Gallentine
The classic Ketogenic diet (KD) is a high-fat, low-carbohydrate treatment that has been used in the treatment of epilepsy for nearly 100 years. When implemented correctly, KD therapy induces a state of ketosis by making dietary fat the main source of energy for the body. This state of ketosis mimics fasting, which has long been association with beneficial effects in epileptic seizures. In the past 20 years there has been a renewed use of diet treatment for epilepsy and development of modifications, which allow more protein and carbohydrate intake and require less fat intake. The newer diet treatments do not always produce the levels of ketosis found in patients on the classic KD, but do maintain a steady blood glucose level, avoiding postmeal glucose rises seen with a typical diet. Despite continued advancements in the development of antiepileptic drugs (AEDs), approximately 30% of patients with epilepsy have refractory seizures, representing a significant need for additional treatment options. Because of this need, the use of metabolism-modifying diets remains a therapeutic option for drug-resistant epilepsy. After careful screening and education, metabolic treatment can be used in children or adults to provide a safe, well-tolerated therapy for many forms of epilepsy. This chapter will discuss some practical aspects of implementing metabolic treatment for epilepsy.
HISTORY
Fasting and other diet-based therapeutic approaches have long been used to treat epilepsy. Fasting is, in fact, the only therapeutic measure for epilepsy recorded in the Hippocratic collection. The origin of modern KD therapy came in 1921, when Dr. Rawle Geyelin gave a presentation at the annual meeting of the American Medical Association in which he reported the outcomes of several children with epilepsy who had benefited from fasting under the care of osteopathic physician Dr. Hugh Conklin. For some of those children the reduction in seizures was long lasting. That same year, the Mayo Clinic Bulletin published the first description of a “ketogenic diet.” In this description, dietary intake was calculated to simulate ketosis, a metabolic state that develops during fasting. This was accomplished by restricting protein and carbohydrate intake and supplying dietary fat as a source of calories, thereby mimicking the body’s use of its own fat during a fasting situation. For the 20 years that followed, primarily because of researchers at the Mayo Clinic, the KD became a popular and well-studied treatment for both children and adults with epilepsy. However, as AEDs were introduced into the United States, use of the KD declined until it was used only at a few academic centers.
The medium-chain triglyceride (MCT) oil diet was developed about 40 years ago to provide a diet approach that produced a similar level of ketosis as the classic KD while allowing for more carbohydrate. In the past 20 years, the Modified Atkins diet (MAD) and Low Glycemic Index Treatment (LGIT) have been developed, which have shown that the fat content of the treatment does not have to be as high as traditionally believed.
Metabolic treatment is currently being used all over the world, and there is a growing scientific interest in the physiological basis of the diets’ beneficial effects. At this writing, a PubMed search revealed that since 1994 over 1,100 peer-reviewed articles have been published on the KD. Randomized, controlled trials and meta-analyses consistently show that about 50% of children respond (ie, at least a 50% reduction in seizures) to this metabolic therapy. There are also indications that metabolic treatment may be effective for adults with epilepsy and in status epilepticus (SE), and it is a first-line treatment of seizures associated with glucose transporter-1 (GLUT-1) deficiency. Given that approximately 200 centers offer this treatment today, it is estimated that about 3000 children may be actively receiving metabolic treatment (1). With the growing use and awareness of metabolic treatment for epilepsy, an expert committee of 26 neurologists and dietitians from 9 countries has recently published a consensus guideline endorsed by the Child Neurology Society. This guideline offers recommendations for optimal management of children on the KD, and it offers clarification on certain aspects of KD implementation that have often diminished efficacy or caused complications in situations where clinicians have not been trained in appropriate diet management (2). The current guideline helps inform decisions on patient selection, pre-KD counseling and evaluation, specific dietary therapy selection, implementation, supplementation, follow-up management, adverse event monitoring, and eventual KD discontinuation.
PHYSIOLOGICAL BASIS FOR THERAPEUTIC EFFECTS
The mechanism of action of the KD in seizure control is not known. Importantly, however, all variants of the KD share the common characteristic of restricting carbohydrate intake and making fat the primary source of energy for the brain. The resulting metabolic state somehow affects neuronal excitability, and thus far the unknown mechanism appears to be unique from the mechanisms of action of other anticonvulsant therapies. Restriction of carbohydrate is clearly critical; it has been reported that children whose seizures were well controlled on KD therapy experienced a recurrence of seizures within 1 hour of intravenous infusion of glucose (3). Emerging data indicate that the beneficial effects of KDs on epilepsy may arise from a variety of different mechanisms that include carbohydrate reduction, activation of ATP-sensitive potassium channels by mitochondrial metabolism, inhibition of the mammalian target of rapamycin pathway, and inhibition of glutamatergic excitatory synaptic transmission. The importance of mitochondria to energy homeostasis makes them an important point of focus in current research on the mechanism of action of metabolic treatment.
EFFICACY
Since the renewal of interest in the KD, hundreds of case reports and prospective studies have yielded very similar results. Approximately 50% to 60% of children experience seizure reduction of at least 50%, and about one-third of children see their seizures reduced by greater than 90% (4). About 10% of children become seizure-free after using the KD, and there is no apparent variation with sex or age of the children (2). Moreover, the response seems to be quite durable, with many children maintaining seizure control over a period of years. In some cases, seizure control even continues after the KD has been stopped. It is also important to note that a randomized controlled trial has published results that were very representative of the overall trends that were drawn from the case reports and prospective studies mentioned earlier (5). In this trial, 103 children who experienced daily seizures despite treatment with at least two AEDs were randomized to the KD or to a control group. After 3 months, 38% of the children in the KD group had greater than 50% seizure reduction, compared to 6% of the control group (P < .0001). Seven percent of the KD group had greater than 90% seizure reduction compared to 0% of the control group (P = .0582). There was no indication of a significant difference in the efficacy of the KD between symptomatic generalized or symptomatic focal syndromes.
Efficacy in Subpopulations: Infantile Spasms
Results from a study of 104 children (mean age 1.2 years) indicate that intractable epilepsy due to infantile spasm (IS) responds very well to KD and suggest the diet may come to be considered as a first-line therapy for IS (6). The children in this study had already been treated with an average of 3.6 AEDs; 71% included corticosteroid or vigabatrin. Using an intent-to-treat analysis, greater than 50% improvement occurred in 64% of the children after 6 months of treatment, and 77% responded after 1 to 2 years. Furthermore, 37% of the children became spasm-free for at least a 6-month period within 2.4 months (median) of starting the KD. Notably, 62% showed improvement in development, 35% had EEG improvement.
Efficacy in Subpopulations: Adults
At present, there are few reports on the use of KD in adult epilepsy, but the available data suggest that the KD successfully reduces seizures in adults (4). Unfortunately, the KD can be challenging to use in adults due to the necessity of making very restrictive lifestyle changes in order to achieve and maintain a therapeutic level of ketosis. However, a MAD has recently shown efficacy in a study of 30 adults (aged 18–53 years) with intractable epilepsy (7). Using an intent-to-treat analysis, 47% of the participants experienced more than 50% seizure reduction after 1 month and 3 months on the MAD. The median time to improvement was 2 weeks (range: 1–8 weeks). After 6 months on the MAD, 33% had greater than 50% seizure reduction; unfortunately, 30% of the participants discontinued the MAD before 3 months. Limited data indicate that long-term use of the Atkins diet in adults with obesity is safe. This observation suggests that use of the MAD for seizure control may be considered for motivated adults.
Efficacy in Subpopulations: Febrile Infection-Related Epilepsy Syndrome
There is mounting evidence appearing in the literature that the KD is effective in cases of febrile infection-related epilepsy syndrome (FIRES), which can be quite difficult to control and can adversely affect development (4). There has been a report on nine children with FIRES who presented in SE that was refractory to conventional treatment (8). The KD was given to these children as a ketogenic formula administered via tube feeding; in seven patients seizures stopped within 2 to 4 days (mean 2 days) after the onset of ketonuria and 4 to 6 days (mean 4.8 days) following the onset of the diet. The patients recovered consciousness within 24 to 48 hour after the seizures had stopped, and they recovered motor function within the following weeks. Six of the responders remained on the KD for 6 months to 2 years (mean 1 year). Within a few months seizures recurred, but they consisted of isolated seizures occurring once or twice a week.
CLINICAL IMPLEMENTATION
Overview
Successfully implementing and maintaining treatment depends on close collaboration of the metabolic team with the patient and family. A skilled dietitian on the team is essential to maintain appropriate dietary and calorie intake. Dietitian support throughout therapy is important to success because small changes in diet composition can correct or prevent issues like weight loss, growth failure, or discontinuation due to prescribed foods becoming boring or unpalatable. In addition to the dietitian, the team typically includes a pediatric neurologist, epilepsy nurse, and pharmacist, all who have knowledge of, and experience with, metabolic treatment. Therapy is usually recommended for at least a 3-month trial. After a patient has completed initiation of the metabolic treatment, fine-tuning may be needed to maximize the diets’ efficacy, ensure good tolerability, detect and minimize complications, and meet changing nutritional requirements to compensate for growth in children. If seizure activity is reduced, the treatment can, in some cases, be weaned in about 2 years. In some patients, weaning may become possible earlier; others may continue on the therapy for many years.
Treatment Options
All variations of metabolic treatment involve specific adjustments in the proportion of total calories from fat, protein, and carbohydrate, which will lead to the lower glucose levels and/or state of ketosis that has been observed in patients successfully treated with diet therapy. The proportion of ketogenic foods (high in fat) to nonketogenic foods (high in carbohydrates and/or protein) is referred to as the diet ratio as seen in Table 32.1. In sharp contrast to the diet ratios seen in the treatment diets, a typical American meal provides about 50% of the calories from carbohydrate, 30% from fat, and 20% from protein. Higher diet ratios correspond to a higher percentage of calories from fat as can be seen in Figure 32.1. The forms of metabolic treatment also vary in their implementation with some requiring carefully weighing foods on a gram scale according to calculated meal plans or in contrast using household measures for food portions and counting grams of carbohydrate and possibly protein and fat to adhere to recommended intake goals. Clearly, effective implementation of this therapy involves significant lifestyle changes and commitment on the part of the patient and family. Therefore, in many cases, the patient or family is involved in the decision regarding the form of metabolic treatment.
TABLE 32.1 Understanding the Ketogenic Diet Ratio
The ratio determines the proportion of calories from ketogenic foods (high % of calories from fat) and nonketogenic foods (high % of calories from protein and/or carbohydrate)
DIET RATIO | FAT GRAMS | PROTEIN AND CARBOHYDRATE GRAMS |
1:1 | 1 | 1 |
2:1 | 2 | 1 |
3:1 | 3 | 1 |
4:1 | 4 | 1 |
For some children, it is important that the diet plan maintains elevated levels of blood ketones. Another important goal is to avoid increases in blood glucose levels after carbohydrate consumption. Figure 32.2 illustrates the respective compositions, or ratios, of different metabolic options currently being used in epilepsy treatment.
![]() The “classic” KD has been used to treat epilepsy since the 1920s. The classic KD is typically 3 to 4 grams of fat (ketogenic) for every 1 gram of protein plus carbohydrate (nonketogenic).
The “classic” KD has been used to treat epilepsy since the 1920s. The classic KD is typically 3 to 4 grams of fat (ketogenic) for every 1 gram of protein plus carbohydrate (nonketogenic).
![]() The MCT diet incorporates MCT oil as a contributor to the total fat intake in the diet. Since medium-chain triglycerides are more ketogenic than other fatty acids, this addition lowers the total amount of fat needed and allows children to eat more protein and carbohydrate with this diet than with the classic KD.
The MCT diet incorporates MCT oil as a contributor to the total fat intake in the diet. Since medium-chain triglycerides are more ketogenic than other fatty acids, this addition lowers the total amount of fat needed and allows children to eat more protein and carbohydrate with this diet than with the classic KD.
![]() The LGIT allows for more carbohydrates than the other three diets. However, all the high carbohydrate foods the child eats must have a low glycemic index. The glycemic index is a measure of how much a particular food raises postprandial blood glucose.
The LGIT allows for more carbohydrates than the other three diets. However, all the high carbohydrate foods the child eats must have a low glycemic index. The glycemic index is a measure of how much a particular food raises postprandial blood glucose.
![]() The MAD, as the name suggests, is a modification of the Atkins diet used for weight loss. When starting the diet, children are allowed about 10 grams while adults are allowed about 20 grams of carbohydrate per day, and they get about 60% of their calories from fat.
The MAD, as the name suggests, is a modification of the Atkins diet used for weight loss. When starting the diet, children are allowed about 10 grams while adults are allowed about 20 grams of carbohydrate per day, and they get about 60% of their calories from fat.
Meals, on all treatment options, will include a source of fat such as butter, oil, mayonnaise, or cream. In addition, meals include meat, egg, or cheese as the protein source. The carbohydrate allotment will typically be a small serving of fruit or vegetable. Since fat provides 9 kcal per gram compared to 4 kcal per gram from carbohydrate and protein, a high-fat diet will include smaller portions of foods as can be seen by comparing the 500-calorie ketogenic meal in Figure 32.3A and the more typical 500-calorie meal in Figure 32.3B.
A flexible approach may also be used to prescribing the treatment diet. The patient may begin treatment with one specific type of diet, but it is also possible for the ketogenic dietitian to combine features from some or all of the diets represented in the Figure 32.2. For example, MCT oil may be added to classic KD or MAD. Use of all metabolic treatments involved the same contraindications and they all require careful monitoring, fine-tuning, and consideration of micronutrient supplementation requirements. Choice of diet plan in some cases may be determined by patient age, dietary preferences, or feeding methods. A pretreatment diet assessment conducted by the ketogenic dietitian is instrumental in weighing the treatment options and determining the appropriate choice for each patient. Patient and family preference must also be strongly considered in the diet decision. Finally, it should be noted that a recent study of 40 patients with symptomatic intractable epilepsy made a comparison between the classic KD (n = 10), the MAD (n = 15), and a normal, unchanged diet (n = 15) (9). They found that the frequency and severity of seizures showed the best improvement in the classic KD patients followed by the MAD group, then the patients on a normal, unchanged diet using AEDs only.
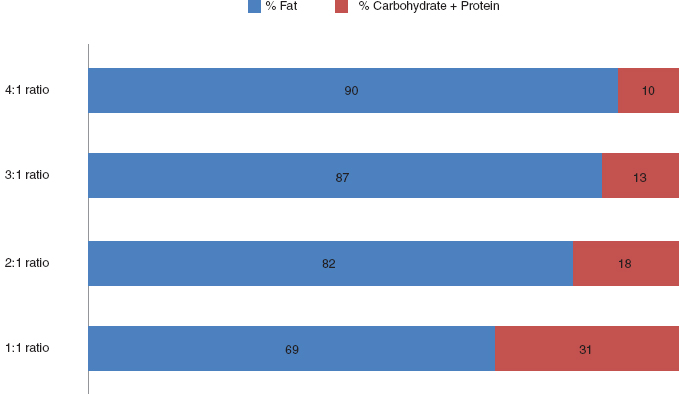
FIGURE 32.1 Relationship of ketogenic diet ratio to percentage of fat to nonfat calories.
![]()
Potential Candidates for Therapy
Metabolic treatment is an established, nonpharmacologic treatment for intractable seizures of various etiologies that is both safe and effective. Data suggest that patients with seizures that are resistant to two AEDs should be considered for metabolic treatment given the diets’ efficacy and the low probability of significant improvement with additional trials of AEDs. Table 32.2 lists indications for metabolic treatment based on the evidence that is presently available (4).
There is also support in the literature for using classic KD therapy in cases of Lennox-Gastaut syndrome (10), and very good response rates have been reported for children with absence epilepsy (11).
Pathway to Initiation of Therapy
As indicated in Table 32.3, starting metabolic treatment requires extensive advance preparation. After a referral has been made to the ketogenic dietitian, actual initiation of the diet is preceded by a thorough nutrition assessment, including a 3-day food record, a review of medications to determine their carbohydrate content, and obtaining some key baseline laboratory values. A specific diet treatment plan is developed based on the child’s age, activity level, route of feeding, developmental level, and current nutrition status.
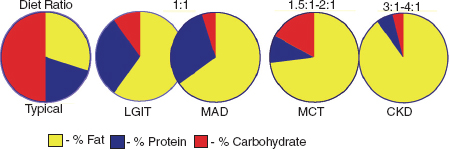
FIGURE 32.2 Comparison of diet ratios and treatment options.
![]()
FIGURE 32.3 Comparison of ketogenic meal (A) to more typically meal (B). Both meals provide 500 calories.
Note: Food portions are small because of the high fat content in the diet. Both of these meals provide 500 calories. Ketogenic meal: 86 grams heavy cream (296 calories); 12 grams broccoli; 34 grams chicken; 20 grams butter. Nonketogenic meal: 240 grams 1% milk (101 calories); 180 grams broccoli; 140 grams chicken (5 grams butter; 25 grams of dinner roll).
![]()
TABLE 32.2 KD Therapy for Seizure Control: Indications and Contraindications
Probable benefit (at least two publications) Glucose transporter protein-1 (GLUT-1) deficiency Pyruvate dehydrogenase deficiency (PDHD) Myoclonic–astatic epilepsy (Doose syndrome) Tuberous sclerosis Rett syndrome Severe myoclonic epilepsy of infancy (Dravet syndrome) Infantile spasms Selected mitochondrial disorders Children receiving only formula (infants or enteral feedings) |
Contraindications Pyruvate carboxylase deficiency Porphyria β-oxidation defects: Medium-chain acyl dehydrogenase deficiency (MCAD) Long-chain acyl dehydrogenase deficiency (LCAD) Short-chain acyl dehydrogenase deficiency (SCAD) Long-chain 3-hydroyacyl-CoA deficiency Medium-chain 3-hydroyacyl-CoA deficiency Primary carnitine deficiency Inadequate ability to maintain nutrition or comply with the KD restrictions Children with clear focal lesion potentially resectable* |
* Relative
Source: Adapted from Ref. (4). Kossoff EH, Wang H-S. Dietary therapies for epilepsy. Biomed J. 2013;36(1):2–8.
TABLE 32.3 Metabolic Treatment Roadmap
1. Referral to ketogenic dietitian. 2. Complete baseline studies. 3. Review of medications for carbohydrate content. 4. Patient or caregiver completion of 3-day food record. 5. Nutrition assessment completed. 6. Determination of metabolic treatment prescription including decision on in-patient or out-patient initiation. 7. Provide patient and/or caregiver education on initiation and management of treatment. 8. Scheduling initiation. 9. Initiation. 10. Monitoring and fine tuning. 11. Discontinuation of treatment. |
Baseline Studies
In addition to the laboratory studies to rule out metabolic disorders that would be a contraindication to use of a high-fat metabolic treatment, Table 32.4 lists some laboratory values that should be assessed before starting the metabolic treatment; these include, but are not limited to, serum glucose, albumin, total protein, fasting cholesterol and triglycerides, and serum chemistries. Also, since metabolic treatment has been associated with an increased chance of kidney stones, it is prudent to find out whether there is a strong personal or family history of kidney stones; a renal ultrasound and nephrology consultation may be needed.
TABLE 32.4 Laboratory Evaluation Prior to Initiating KD
Serum amino acids Serum ammonia Serum acylcarnitine profile Lactic acid Urine organic acids Complete blood counts with platelets Electrolytes including serum bicarbonate, calcium, zinc, selenium, magnesium, phosphorus Serum liver and kidney tests Fasting lipid profile Urinalysis Urine calcium and creatinine Antiepileptic drug levels (if applicable) |
Review Carbohydrate Content of Medications
The strictly controlled dietary intake of metabolic treatment leaves little room for the additional carbohydrates that may be found in medications. An upper limit of one gram of carbohydrate per day from medications and dietary supplements has been used by the authors. Patients who have responded well to metabolic treatment, but then exceed their daily carbohydrate limit, risk having a relapse of seizure activity. After excess carbohydrate intake it can take 2 to 3 days for metabolism to return to the preexposure state. Table 32.5 provides some examples of how medications may contain amounts of carbohydrate that are quite significant when considered in the context of a diet that calculates daily carbohydrate intake down to the milligram. It is important to note that metabolic treatment is often initiated after the patient has failed two or three anticonvulsants, and is therefore likely to be initiated while the patient is on multiple medications. Pharmacists can serve an important role in helping minimize medications that have high carbohydrate content and avoid this error in the management of therapeutic ketosis.





