Fig. 1
The plate illustrates T. cruzi circulating in the blood (a); pseudocyst formation (b); lesions in cardiac fibers (c); healing with fibrosis (d)
Besides direct parasite infection, another main mechanism involved in the pathogenesis of Chagas disease is represented by autoimmunity induced by T. cruzi and its products. This mechanism, introduced by Santos-Buch and Teixeira (1974) and Teixeira (1975), and then investigated in several other studies, is complex and controversial. In recent years, the involvement of autoimmunity in Chagas disease has been critically reconsidered especially by Tarleton (2003), and even Teixeira and coworkers (2006), who have stated that this mechanism is still “open for investigation”.
In a discussion on the evolution of chronic Chagas cardiopathy, Higushi (1999) took the view that CD4+ and CD8+ T lymphocytes are sensitized by T. cruzi and its antigens, with development of anti-myocardial T cells, macrophage activation and platelet aggregation. This may lead to ischemia, development of chronic Chagas myocarditis, impairment of the ANS, arrhythmia, cardiac dilatation and death due to ventricular fibrillation and/or heart failure.
In pathological studies on the digestive and cardiac forms of chronic Chagas disease, Köberle (1961, 1968) showed that megaesophagus and megacolon are consequences of denervation of the myenteric plexuses of these organs due to an inflammatory reaction induced directly by T. cruzi, and that impairment of the parasympathetic nervous system is involved in cardiopathy, as initially described by Vianna (1911). Consistently with this interpretation, denervation of the heart, esophagus, colon and other hollow viscera has been repeatedly considered responsible for chronic Chagas disease, produced directly by the inflammatory reaction caused by T. cruzi (see, for example, Prata 2001; Teixeira et al. 2006).
Molecular mechanisms potentially involved in neuronal damage and repair in T. cruzi infection, as well as the potential role of parasite-derived neurotrophic factors in the interplay between the parasite and the ANS have recently been reviewed (Chuenkova and Pereira-Perrin 2011). Concerning Chagasic cardiopathy, parasympathetic denervation of the heart, with loss of ganglion neurons and vagal nerve terminals, is an early event in T. cruzi infection. As the infection progresses, damage of the sympathetic nervous system also occurs and targets post-ganglionic nerve terminals in the heart rather than neurons in the cervical and stellate ganglia.
Neurodegeneration in the gastrointestinal tract targets the extrinsic innervation (neurons of the parasympathetic ganglia) and intrinsic innervation. Of special interest is the involvement of the enteric nervous system. Composed of millions of neurons, this system subserves the extensive intrinsic innervation of the gut comprising the myenteric plexus (of Auberbach) and the submucosal plexus (of Meissner). Enteric neurons comprise a variety of phenotypes in terms of neurotransmitters and neuromodulators, and control gastrointestinal motility, secretory processes and nutrients, as well as local blood flow (Furness 2012). Loss of enteric neurons in Chagas disease targets especially inhibitory neurons containing nitric oxide synthase and vasoactive intestinal peptide, possibly due to molecular mimicry between T. cruzi and enteric proteins (Rivera et al. 2011).
Puzzling issues are also represented by the fact that about 70 % of T. cruzi-infected individuals are asymptomatic despite retaining pathogenic parasites, and by the long latency (20–30 years) of the onset of chronic disease after the initial acute infection (Chuenkova and Pereira-Perrin 2011). Parasite-host interactions, including neuroregenerative responses of PNS and/or ANS fibers to the infection, diversity in virulence and tissue tropism of T. cruzi strains have been implicated in these enigmatic phenomena.
3.1 Biological Diversity of T. cruzi Strains and Organ Tropism
The etiological agent of Chagas disease exhibits great genotypic and phenotypic diversity with different biological properties, virulence and geographical distribution. The same host may be simultaneously infected by different T. cruzi strains (Devera et al. 2003). Classifications of the parasite heterogeneity have been proposed since decades. In the 1960s, when the present molecular tools were not available, Coura et al. (1966) proposed the designation of “cruzi complex” on the basis of morphological, immunological and pathogenic diversity (see also Devera et al. 2003). Subsequently, T. cruzi have been classified into “biodemes” according to biological variations (Andrade and Magalhães 1977), “zymodemes” according to isoenzyme variability, (Miles et al. 1980), and “schizodemes” according to molecular diversity (Morel et al. 1980). More recently, on the basis of genetic diversity T. cruzi have been partitioned into six groups, renamed by consensus as TcI- TcVI (Zingales et al. 2009, 2012), integrating the above-mentioned variations.
Heterogeneity of T. cruzi strains has been repeatedly implicated in the different presentations of Chagas disease and in the infection targeting of different organs. For example, the classification of parasite strains into three “biodemes” (types I, II, III) has taken into account tissue tropism together with strain morphology, virulence and pathogenicity (Andrade 1976; Andrade and Magalhães 1977). After T. cruzi inoculation in mice, type I shows a tropism for macrophages in the initial phase of the infection, with high virulence and parasitemia peaking 7–12 days after inoculation, predominance of slender forms of T. cruzi, and 100 % mortality of the mice on the 12th day after infection. Infection with T. cruzi type II involves myotropism, predominantly for the myocardium, during the acute phase, and a greater number of parasite wide forms, with parasitemia peaking from 12 to 20 days after infection. Type III infection involves tropism for skeletal muscles, with predominance of the parasite wide forms, parasitemia peaking 25–30 days after infection, and low mortality among the infected animals.
The genetic variation of strains and clones of T. cruzi has been correlated with specific receptors in the host’s organs or systems, and it has been hypothesized that this could account for the parasite tropism for a given organ (Macedo and Pena 1998). According to this interpretation, different clonal-histotropic strains of T. cruzi would have affinity for different receptors expressed in cardiac or gastrointestinal tract tissue, so that Chagas disease pathology would be determined by host-parasite genetics. This theory, however, has not received experimental confirmation.
4 Involvement of the Nervous System in the Acute Phase of Chagas Disease
The involvement of the CNS in the acute phase of Chagas disease has been reported since the initial descriptions of the disease (Chagas 1911, 1913, 1916), especially meningoencephalitis, which is severe and fatal in infants (2–4 years of age). This is characterized by headache, vomiting, mental confusion, convulsions, meningeal signs and presence of T. cruzi in the cerebrospinal fluid. These patients present cranial hypertension, with elevated proteins and predominance of lymphocytes in the cerebrospinal fluid. In fatal cases, lesions disseminated in the leptomeninges and CNS have been reported, especially in the grey matter, with nodular encephalitis in multiple foci, diffuse vasculitis and nests of parasites in astrocytes and microglia. These lesions, originally described by Vianna (1911), have been repeatedly documented in subsequent studies (see, for example, the reviews by Sica 1994; Pittella 2009). Brain lesions with cognitive and behavioral disturbances as sequels have been reported in children of 6–12 years of age who had suffered acute meningoencephalitis due to T. cruzi infection (Sica 1994).
The involvement of the heart in the acute phase of Chagas disease affects not only cardiac myocytes but, as mentioned above, also the ANS. Damage to the parasympathetic and sympathetic innervation of the heart leads to atrio-ventricular (AV) block, supraventricular and ventricular extrasystoles and branch block of the bundle of His and Purkinje fibers. In cases of acute Chagas disease, ventricular and supraventricular extrasystoles have been reported in 38.5 % of the patients, and first and second-degree AV block in 30.8 % of the patients (Pinto et al. 2008).
Two other conditions that may lead to involvement of the CNS in acute Chagas infection are congenital transplacental transmission and immunosuppression. Transplacental transmission of T. cruzi may lead to abortion, prematurity and organ lesions, including premature death due to CNS involvement, or may lead to mental retardation of the survivors. Congenital Chagas infection was first recorded by Carlos Chagas himself (1911, 1913). He observed two newborns with episodes of convulsions who died on the 6th and 8th days of life and whose necropsies revealed the presence of T. cruzi in the CNS. Although infrequent, cases of congenital Chagas infection have been described in all endemic countries in South America (Freilij et al. 1994). Seminal pathological studies on the congenital form and on Chagas placentitis have been conducted by Bittencourt and coworkers (Bittencourt 1963; Bittencourt et al. 1972).
Immunosuppression (especially since the emergence of HIV-AIDS) makes the CNS a preferential target for Chagas infection, in the form of meningoencephalitis and focal lesions, similar to those produced by toxoplasmosis. In immunosuppressed patients Chagasic encephalitis can also occur in multiple foci with necrotizing features, or in tumoral or psedutotumoral forms (brain “chagoma”). Besides HIV-AIDS, other causes of immunosuppression and reactivation of acute Chagas disease include malignant tumors and chemotherapeutic treatment, as well as organ transplantation, which entail serological and parasitological investigations. Immunosuppressive drugs include azathioprine, corticosteroids, cyclosporins or immunosuppressive monoclonal antibodies and anti-lymphocytic serum, Concerning organ transplantation, a protocol for the control of organ donors and recipient patients before and after transplantation has been established taking into account the following points (1) assessment on live or dead donor; (2) assessment on Chagas disease recipient; (3) post-transplantation control; (4) follow-up for presence of parasites after transplantation; and (5) treatment and follow-up of patients 30, 60 and 90 days after the treatment to assess the parasitological cure (Freilij and Storino 1994).
5 Nervous System Involvement in the Chronic Phase and Forms of Chagas Disease
The chronic phase of Chagas disease begins by definition 60–90 days after the acute phase, when parasitemia has greatly reduced due to the action of circulating antibodies. Acute manifestations of the disease are no longer apparent and T. cruzi are no longer detectable in the bloodstream by means of direct methods (fresh blood examination and concentration methods such as microhematocrit).
The chronic phase of Chagas disease may present in four distinct clinical forms mostly due to different degrees of PNS and ANS impairment (a) indeterminate form, without evident signs and symptoms, and with normal clinical examination and electrocardiographic and radiological examinations of the heart, esophagus and colon; (b) cardiac form, with damage of various severity of the heart and its autonomic innervation; this form may also lead to sudden death; (c) digestive form, with damage of various severity of the gastrointestinal tract, which may lead to dysperistalsis of the esophagus with dilatation and functional alterations of different severity, and megacolon; (d) mixed cardiac and digestive forms (Coura 2007). These forms are dealt with below.
Impairment of the PNS, with sensory disturbances, has also been reported in more than 10 % of the cases of chronic Chagas disease (Genovese et al. 1996). Electromyographic studies on such cases have shown reductions in motor and sensory conduction, including in cases of chronic indeterminate form of the disease.
Controlled electromyographic studies on different muscles, conducted in Chagas and non-Chagas disease patients in Brazil (De Faria et al. 1977) and Argentina (Sanz et al. 1978), have demonstrated muscle motor denervation in patients in the chronic phase of Chagas disease. This has been proven by histopathological and histochemical findings (Sica 1994).
5.1 Chronic Indeterminate Form with Mild Involvement of the Autonomic Nervous System
The chronic indeterminate form of Chagas disease is asymptomatic and initiates, by definition, just after the acute phase. It is characterized by low parasitemia and high antibody levels, and no clinical manifestations are demonstrable through routine clinical examinations. Electrocardiographic and radiographic examinations of the heart, and radiological features of the esophagus and colon are normal. After the acute phase, the majority of the patients enter this form of the disease, and they may remain affected for one to two decades or for the rest of their lives. However, about 30 % of the cases may evolve to the chronic cardiac or digestive form. Although asymptomatic, the chronic indeterminate form may show ventricular extrasystoles and monometric alterations at examinations with sensitive methods, such as dynamic electrocardiography (Holter). These abnormalities result from sparse inflammatory foci, including localized neuronal lesions (Dias and Macedo 2005). Pathological studies on patients with the indeterminate form of Chagas disease who suffered unexpected or violent sudden death have shown myocardial abnormalities, including damage of the intracardiac autonomic innervation (Lopes et al. 1975, 1981) (Fig. 2).
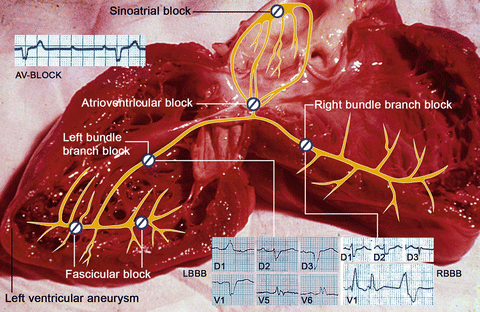

Fig. 2




Autoptic specimen of the heart of a patient affected by chronic Chagas cardiomyopathy, who died due to heart failure. The superimposed drawing and electrocardiographic charts show: Sinoatrial block; complete atrioventricular (AV) block; right bundle branch block; left bundle branch block; fascicular block and left ventricular aneurysm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








