Fig. 1
(a) “Kilo” of khat. “Kilo” is not a weight reference but a term used for a bundle. Three to four bundles can be consumed per day or more when there is khat party or binging. (b) The leaves and the bark of the twigs are chewed and tucked into the cheek. (c) Muguka, leaves from different part of Catha edulis, cheaper than miraa and the use of which has increased in recent times
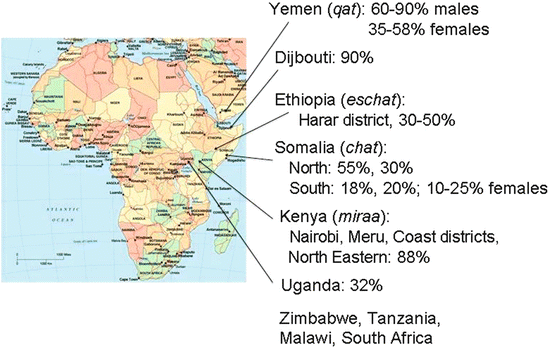
Fig. 2
Major areas of khat use along the eastern Africa and Arabian Peninsula with local names for khat and available estimated percentage of users. (Compiled from various sources)
Several early travellers to the Arabian Peninsula commented this habit.
In the 18th century, Neibuhr wrote “…never saw the Arabian use opium like the Turks and Persians. Instead of taking this gratification, they chew kaad [khat]. These are buds of a certain tree, which are brought in small boxes from the hills of Yemen.” In early 19th century, Abdullah bin Adbul Kadir (1854), a traveller from Malay, described the prevalence of khat chewing in Al Hudaydah, Yemen: “You observed a new peculiarity in this city—everyone chewed leaves as goats chew the cud. There is a type of leaf, rather wide and about two fingers in length, which is widely sold, as people would consume these leaves just as they are; unlike betel leaves, which need certain condiments to go with them, these leaves were just stuffed fully into the mouth and munched. Thus when people gathered around, the remnants from these leaves would pile up in front of them. When they spat, their saliva was green. I then queried them on this matter: ‘What benefits are there to be gained from eating these leaves?’ To which they replied, ‘None whatsoever, it’s just another expense for us as we’ve grown accustomed to it’. Those who consume these leaves have to eat lots of ghee [clarified butter] and honey, for they would fall ill otherwise. The leaves are known as Kad [khat].” These and other observations are similar of the use of coca leaves among the people of western South America.
In 1909, the epidemic of opium use in China resulted in the Shanghai conference, which led to the 1912 International Opium Convention of The Hague, the start of the international regulations in the trafficking, trading and controlling access to drugs of abuse. This convention was subsequently taken up by the League of Nations, and later by the United Nations. In 1935, the matter of khat use and its regulation was first discussed, and debated on and off for a number of years, ultimately cumulating in the resolution that khat use was a regional issue and as confined to a few countries (WHO 1964), possibly not an issue of international regulation, but one that needed to be studied further.
In the deliberations on whether to enter khat on the list of internationally controlled substances, it was recognized that its active ingredient had to be isolated, identified, and its abuse potential studied before a decision could be made. In the 1930s, Wolfes had isolated cathine (d-norpseudoephedrine), but subsequent studies showed that cathine could not fully account for the activity of khat in the user. The United Nations Narcotics Laboratory, after studying fresh khat samples from different sources, isolated 20 components, which included a large group of alkaloids (cathedulins), and in particular, isolated cathinone (alpha-aminopropiophenone), a liable substance, which was a more active ingredient of khat than cathine (UNODC 1980). Once cathinone has been isolated, synthesized and its molecular structure determined (Schorno and Steinegger 1978; Braendan 1979) it was found to be similar to amphetamine, both belonging to the phenethylamine group (Fig. 3). In 1980, the World Health Organisation (WHO) classified khat as a drug of abuse that can produce mild to moderate psychological dependence. Currently, khat and cathinone are on the list of banned or controlled substances in several countries and khat has a higher international profile due to the spread of its use to parts of the world where it was not originally used.
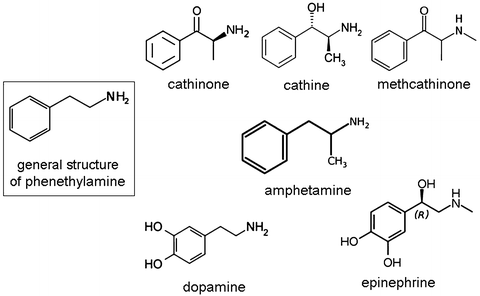

Fig. 3
Phenethylamine group of molecules: note that the addition or substitution on the phenyl ring, ethyl chain or amino group of the molecule produces a large group of psychoactive and bioactive molecules including cathinone and cathine, which are active ingredients found in khat
3 Pharmacological Effects on the Dopamine, Serotonin, and Norepinephrine Systems
Two widely used techniques to determine where in the brain a drug could affect neurotransmitter release are in vitro tissue and in vivo microdialysis methods. Of the number of neurotransmitters systems in the brain, studies on drugs of abuse have highlighted the role of three: dopamine, serotonin, and norepinephrine (noradrenaline). In an in vitro study of rabbit striatal and rat nucleus accumbens tissue pre-labeled with 3H-dopamine, there was increase in the release of radioactivity on cathinone application (Kalix 1986; Kalix et al. 1987). However, without further analysis, it is not possible to conclude if the increased radioactivity released from these tissues was due only to dopamine or a mixture of dopamine and its metabolites. In in vivo microdialysis studies of the anterior caudate-putamen and nucleus accumbens, (−)-cathinone and (+)-amphetamine increased dopamine levels in a dose-dependent manner with amphetamine having a higher effect at the largest doses used, but at lower doses the amphetamine difference was only seen in the nucleus accumbens (Pehek et al. 1990). In synaptosomal preparation, d,l-cathinone like d-amphetamine, released and blocked uptake of 3H-dopamine, and with repeated high doses of d,l-cathinone there was long-lasting dopamine depletion in various rat brain regions and decreased number of dopamine uptake sites similar to amphetamines, but regional brain levels of norepinephrine and serotonin were not altered (Wagner et al. 1982; Fleckenstein et al. 1999). Cathinone also inhibited the firing of dopaminergic neurons (reversible with haloperidol) in the substantia nigra pars compacta, with potency similar to amphetamine (Mereu et al. 1983). These studies, along with the drug discrimination studies (described below), further emphasized the role of dopamine in the action of cathinone, but this is probably only a part of its mechanism. Dopamine systems are involved in motor function through the striatal tissue and “mental” reward system involving the nucleus accumbens. Hence, increase in dopamine could affect both motor and mental function, though cathinone appears to have a less disruptive effect on motor behavior compared to amphetamine.
Like amphetamine, but a third less potent, cathinone induces release of 3H-serotonin (5-HT) from rat striatal preparations (Kalix 1984) and has four times higher affinity for serotonin receptor in an isolated rat stomach fundus preparation (Glennon and Liebowitz 1982). Repeated high doses of cathinone do not alter regional brain levels of serotonin (Wagner et al. 1982). However, high-dose administrations of cathinone to striatal synaptosomes obtained from drug-treated rats rapidly decreased serotonin transporter function, which should result in higher serotonin levels in vivo (Fleckenstein et al. 1999). Thus, while, in vitro studies show that cathinone causes release of serotonin and has higher binding affinity to peripheral serotonin receptors compared to amphetamine, serotonin is not found to be involved in studies of cathinone’s discriminative mechanism (discussed in later section), but this does not rule out serotonin’s involvement in other effects of cathinone.
While regional brain levels of norepinephrine (noradrenaline) or serotonin are not altered on a long-term basis by repeated administration of d,l-cathinone (Wagner et al. 1982), there is increased release of radioactivity from rabbit heart atria pre-labeled with 3H-norepinephrine (Kalix 1983). The effect on rat right ventricle may involve competitive blockade of norepinephrine transporter rather than simple displacement of norepinephrine (Cleary et al. 2002). These changes in peripheral neurotransmitters are not unexpected as khat, cathinone, and cathine produce sympathomimetic effects in the user, but whether the central neural mechanism involve norepinephrine is not known.
4 Experimental Studies on Behavior and Potential for Addiction
4.1 Behavioral Studies
Like amphetamine, cathinone or khat extract produce psychomotor sensitization -hyperlocomotion (Kalix 1980a; Calcagnetti and Schechter 1992a; Banjaw et al. 2005), behavioral sensitization (Banjaw and Schmidt 2005)—as well as pre-pulse inhibition (Banjaw et al. 2005), and increased isolation induced aggression in rats (Banjaw et al. 2006). The psychomotor sensitization reflects nucleus accumbens involvement (Wise and Bozarth 1987) and the psychostimulant-induced activity is blocked by the dopamine release inhibitors CGS 10746B and isradipine (Calcagnetti and Schechter 1992b).
Like other psychostimulants, cathinone produces a hyperthermic response (Kalix 1980b), which is linked to the neurotoxic effect of amphetamines. Treatment of rats with khat extract produces seizures and decreases seizure threshold elicited by pentylenetetrazol (Oyungu et al. 2007, 2009). In addition, cathinone induces head-twitch response (Connor et al. 2002), which is a behavioral proxy for serotonin receptor 5-HT2A activation (Schmid and Bohn 2010). This is one of the serotonin receptors postulated to be involved in the mechanism of activity of hallucinogens (Vollenweider 2001). Taken together, these findings suggest that khat activity could have some other different and subtle effects compared to amphetamines.
4.2 Addictive and Abuse Potentials
4.2.1 Self-administration and Self-reinforcing Experimental Studies
A routinely used experimental approach to assess whether a drug has addictive and abuse potential is to avail the drug to laboratory animals and observe whether they will self-administer the drug. The frequency and amount of the self-administration gives an indicator of the reinforcing property and abuse potential of the drug.
Using a self –administration continuous set-up, Yanagita (1979, 1986) found in rhesus monkeys a spree type usage of cathinone, as seen with cocaine, with spree periods of 6–59 h. Compared to amphetamine and cocaine, both d,l-and l-cathinone maintained significantly higher rates of responding with l-cathinone being more potent (Schuster and Johanson 1979). Woolverton and Johanson (1984) found the reinforcing effects of d,l-cathinone were comparable to cocaine. In mice (Kuz’min and Evartan 1991), comparison of the pattern of intravenous self-administration of morphine, cocaine, amphetamine, cathinone, and ephedrine produced similar bell-shaped concentration curves typical of compounds with addictive potential. Rats also demonstrated cathinone self-administration, which was increased by block of the dopamine D1 receptor with SCH 2390 but not with D2 receptor antagonist, spiperone, suggesting that dopamine D1 receptors are involved in cathinone’s reinforcing effects (Gosnell et al. 1996).
4.2.2 Conditioned Place Preference
In conditioned place preference (CPP), an experimental approach to assess the abuse potential of a drug, rats given cathinone either by the intravenous (iv) or intracerebroventricular (ICV) route showed preference for the environment to which they were exposed to with cathinone (Schechter 1991a) and this effect was dose dependent (Schechter and Meehan 1993). Interestingly, no tolerance to CPP was found with repeated cathinone administration unlike that observed in drug discriminative behavior (Schechter and McBurney 1991). CPP was attenuated or blocked by pretreatment with dopamine release inhibitor, CGS 10746B (Schechter 1991a; Calcagnetti and Schechter 1993).
4.2.3 Drug Discrimination Experimental Studies
While self-reinforcing and CPP studies provided evidence that cathinone has addictive and abuse potential, these experimental approaches cannot answer the question of whether (1) the feeling produced in an animal (interoceptive cue) with cathinone is similar to other drugs, especially other psychostimulants, and (2) if mechanism of action of cathinone is similar to that of other known psychostimulants. In the drug discrimination experimental paradigm, the animal is trained to perform a particular task under the influence of a drug and another in the absence of that drug. In a two-lever food-motivated operant task, animals press one lever when in the drug induced mental state (interoceptive cue) and the other when not. The drug used for the training is the training drug, and once the animal has learnt the task it is given other test drugs, and if the animal presses the levers correctly, it is assumed that the interoceptive cue induced in the animal by the test drug is similar to that produced by the training drug.
Table 1 summarizes the drugs that have been found to be able to replace or generalize for the cathinone cue and those that could not. The drugs that can substitute for cathinone are known psychostimulants, and cathinone can also substitute when amphetamine or cocaine is used as the training drug. These results support the view that cathinone is a psychostimulant and represents a “natural” amphetamine (Kalix 1992). When cathinone was used to substitute for amphetamine as the test drug, it was found to be twice as potent as amphetamine (Rosecrans et al. 1979). In rats, injecting cathinone into the nucleus accumbens produced discriminative behavior at a much lower concentration than either by intraperitoneal or ICV route, indicating that one site of action of cathinone in the brain is the nucleus accumbens (Schechter et al. 1992).
Table 1
Drugs that can or cannot substitute for cathinone in the drug discrimination experimental paradigm
Can | Cannot |
|---|---|
d-amphetaminea,b,c | Apomorphinea,d,c |
Cocainea, e | Fenfluraminea |
Pripradola | Fentyamyla |
Cathinea | Phydroxyamphetaminea,f |
Methamphetaminee | Phenylethylaminea,d |
Methylphenidatea | Deuterated phenylehylaminee,d,g |
Chlorodiazepoxidea |
In the drug discrimination studies, the time-course for cathinone interoceptive cue behavior in rats was earlier than amphetamine (5 vs. 15–30 min) and effective for about 1 h (Schechter 1989).
Positive results were found in all drug discrimination studies in which the role of dopamine as part of cathinone’s mechanism of action was tested, i.e. the dopaminergic system is involved as reported for other psychostimulants. However, depending on the agent used, the assessment of the involvement of the dopaminergic system in cathinone’s effect differs. Unlike amphetamine, with haloperidol, a dopamine antagonist, Goudie et al. (1986) found at most 50 % reduction in the cathinone cue, and Rosecrans et al. (1979) found that it did not affect the generalization of amphetamine stimulus to cathinone. Pretreatment with haloperidol failed to alter the stimulant properties of cathinone but did partially antagonize those of amphetamine and cocaine (Huang and Wilson 1986) and attenuated cathinone discrimination (Schechter 1986c). However, co-administration with CGS 1074613, a dopamine release inhibitor, totally antagonized cathinone’s generalization to amphetamine (Schechter and Bojaw 1988) and pretreatment blocked cathinone discrimination (Schechter 1992). Use of serotonin antagonist, BC 105/B (Rosecrans et al. 1979), 5-HT receptor blocker, pirenperone (Schechter 1986a), 5-HT3 receptor antagonist, MDL 72222 (Schechter 1992) or inhibiting serotonin synthesis with p-chlorophenylalanine had no effect on the cathinone discrimination in rats (Schechter 1991b). Phenoxybenzamine, an alpha-adrenergic antagonist, also had no effect (Rosecrans et al. 1979). Thus, at least from drug discrimination behavior studies, alteration of the dopaminergic neurotransmitter system appears to play a major role in the mechanism of action of cathinone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








