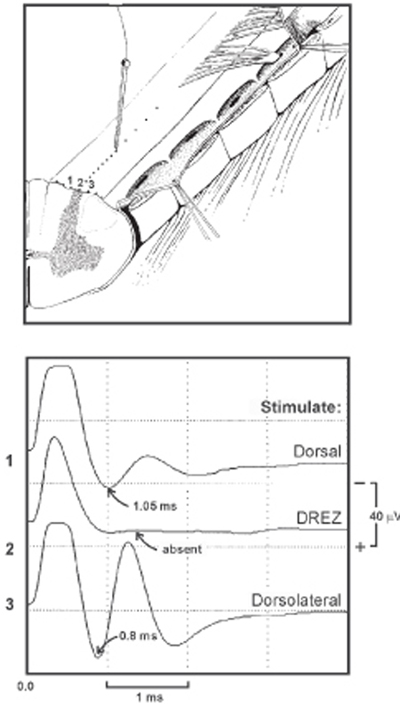
10 Dorsal Root Entry Zone: Lesioning for Intractable Deafferentation Pain A 20-year-old man was involved in a motorcycle accident and suffered a closed head injury with loss of consciousness, multiple extremity fractures, as well as motor paralysis of the right upper extremity. As the patient recovered from his head injury he began to complain of severe pain affecting the right arm. He described his pain as excruciating and of a burning nature, with no significant response to analgesics. He had clinical evidence of brachial plexus avulsions affecting C5 to T1 nerve roots with a completely flail arm. He underwent cervical myelogram and computed tomographic (CT) scan, which confirmed the diagnosis of multiple brachial plexus nerve root avulsions. Multiple medical therapies using different analgesics and antidepressants were not effective to control his pain. Two years following his injury he was eventually referred for dorsal root entry zone (‘DREZ) coagulation. This procedure was performed with no complication, and the patient remained pain free at his 1-year follow-up exam. Deafferentation pain from brachial plexus avulsion In 1974 Sindou et al presented a neuroanatomical study of the DREZ of the spinal cord and suggested lesioning procedures of the dorsal root at its entry into the spinal cord for the treatment of peripheral origin pain and spasticity. The pathophysiological rationale relates to pain generation from deafferentated cells of the substantia gelatinosa of the spinal cord, which develop a type of denervational hypersensitivity, leading to spontaneous firing and increased activity along pain pathways. In 1976 Nashold et al described radio frequency–induced coagulation of the DREZ for the treatment of pain related to cervical root avulsion. This destroys the upper five or six Rexed layers in the dorsal horn. Pain syndromes after brachial plexus trauma can vary tremendously in their extent and different characteristics. Dependent on the force and injury mechanism of brachial plexus injury, two different pain syndromes can develop: (1) neuropathic pain, of peripheral origin, or (2) deafferentation pain, of central origin. The latter syndrome occurs only after root avulsion. However, central pain from root avulsion that is refractory to conservative treatment can be treated neurosurgically with high success rates. If there is stretch without rupture of the nerves, a neuroma in continuity will form. Also, rupture of nerves with intact roots leads to neuromas. However, only some of these neuromas become painful. They can be conceived as pathological nociceptors that develop ectopic foci of hyperexcitability, thus influencing pain pathways. After time, central changes (central sensitization) take place, which will complicate treatment. Nevertheless, this pain is considered to be of the peripheral neuropathic type. Surprisingly, a fair amount of brachial plexus patients without root avulsion will not present with pain being their major complaint. Often they can cope with the pain quite well and some of them do not even regard pain medication as indicated. Furthermore, neurolysis and reconstructive surgery sometimes reduce some of the neuropathic pain to a tolerable level. When a neuroma has been resected and stumps are bridged by grafts, outgrowing axons find a path, which reduces further neuroma formation, and often the associated resulting pain. If neuropathic pain in these cases persists, it can usually be relieved pharmaceutically. At first, common nonsteroidal antiinflammatory drugs (NSAIDs), tricyclic antidepressants, and narcotics should be prescribed. Dosages have to be increased very gradually and often different substances of the same group are tried. If conventional analgesics do not help, the antiepileptic drugs carbamazepine and gabapentin are used with varying success. These have a potential to relieve paroxysmal and burning pain. Due to sporadic side effects, patients need to adjust to these latter drugs. Initial sleepiness and a feeling of being “out of it” will usually disappear. Patients need to be reminded that these medications can only be effective if constant bloodlevels are maintained, which necessitates regular dosing. They are not supposed to be used as on demand analgesics. The therapeutic levels, however, are individually variable. As in epileptic patients, liver and kidney parameters will have to be monitored routinely. The second type of pain that will be encountered in plexus injury is deafferentation pain, a central pain due to root avulsion. Pain is of a significantly higher intensity after root avulsion. Severe, persistent brachialgia is usually indicative of multiple root avulsions. The pathophysiological mechanism of pain generation differs from neuromatous pain, yet the initial pharmacological attempts are along the same line. Deafferentated cells of the substantia gelatinosa of the spinal cord develop a type of denervational hypersensitivity, leading to spontaneous firing and increased activity along pain pathways. Central neurons, in which activity leads to the sensation of pain, develop an enhanced response to input from non-nociceptive afferents. History will quite easily elucidate the cases of deafferentation pain. Pain is characteristically described as burning or crushing or as electrical paroxysm of sharp, shooting, or stabbing quality and may be agonizing. The most severe pain component quite often has a paroxysmal character, with another, underlying severe and persistent dull pain component. In large series, the number of root avulsions seemed to correlate with pain intensity, although pain distribution and dermatome of avulsed root do not always correlate. Weather changes, local cooling, and immobility of the limb are oftentimes aggravating. Local manipulation of the shoulder and arm, activity, and distraction sometimes give slight relief, but in other cases pain may be triggered by direct contact with the arm or exacerbated by cold damp water, infection, or other illness. Onset of deafferentation pain may be immediate, may develop within several days of trauma, or may occur after a delay of several months. Fortunately, many brachial plexus avulsion patients with early and severe pain will improve over time and not develop the intractable central deafferentation syndrome. In up to 25% of patients, the pain subsides to tolerable levels during the first year. Management efforts include pharmacological treatment with narcotic and nonnarcotic analgesics, anticonvulsants, and tricyclic antidepressants. But up to one third of avulsion injury patients continue to have significant pain 2 years later, and these patients are excellent candidates for DREZ lesioning. The midcervical and upper thoracic spinal cord segments are exposed by a laminotomy (followed by laminoplasty) technique to prevent delayed swan-neck deformity. Arachnoidal adhesions are sharply released under operating microscopic magnification after opening the dura. The patient receives pharmacological paralysis with pancuronium intraoperatively to obliterate electromyographic responses during evoked potential recording. A warming blanket is used to reduce loss of temperature, but mere exposure of the spinal cord as well as the use of irrigation during procedures results in subnormal spinal cord temperature, which affects evoked response to some degree. The recording electrode (a silver ball electrode 1 mm in diameter, insulated except for the ball) is placed over the dorsolateral or dorsal aspect of the spinal cord at the rostral or caudal end of the exposure. A needle electrode is placed lateral to the silver ball electrode in the paraspinal muscles to serve as the reference electrode. The stimulation electrode used is a modified cordotomy needle with a diameter of 200 A (insulated except for the 2.5 mm tip) connected to a constant-current unit and a stimulator. A metal plate is attached to the patient’s arm, serving as the reference electrode. A 100 msec square-wave pulse and a stimulation rate of one stimulus per second are used. Responses are amplified by amplifier with a gain of 100,000 and a passband of 3 to 3000 Hz. Responses are averaged using a computer with an analog-to-digital converter and a sampling rate of 51.2 kHz. Typically, 5 to 10 responses are included in each average. Response conduction velocity is calculated by measuring the distance between the stimulating and recording electrode and divided by the latency to the initial negative deflection of the response. Response amplitude is measured as the peak-to-peak voltage of the response. Initially the stimulating electrode is placed where the spinal cord anatomy appears normal (the level at which the dorsal root filaments are intact and can be seen). The stimulating needle is initially placed over the pia-arachnoid, between the denticulate ligament and the posterior lateral sulcus (the DREZ), in a location that should be directly over the lateral corticospinal tract and dorsal spinocerebellar tract. The intensity of the stimulation is gradually increased until a spinal cord evoked potential with an amplitude of ˜10 to 20 mV is elicited. The stimulus intensity necessary to evoke this response is termed the baseline stimulus intensity. Then the stimulating electrode is gradually moved dorsally toward the posterolateral sulcus (DREZ) in ˜1 mm steps. Stimulation and recording are repeated at each step using the baseline stimulus intensity. This procedure is performed under an operating microscope so that the placement of the stimulating electrode can be well visualized. Fig. 10–1 illustrates three different stimulation sites resulting in three different evoked potentials. Dorsolateral spinal cord stimulation (corticospinal tract) evoked a larger response with higher conduction velocity (˜62 m/s) than dorsal spinal cord stimulation (˜41 m/s). The evoked response is absent when the DREZ is stimulated.
 Case Presentation
Case Presentation
 Diagnosis
Diagnosis
 Anatomy
Anatomy
 Characteristic Clinical Presentation and Differential Diagnosis
Characteristic Clinical Presentation and Differential Diagnosis
 Management Options
Management Options
 Surgical Treatment
Surgical Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



