EPIDEMIOLOGY
Age of onset for dementia is typically between 50 and 55 years with a median age of onset of dementia of 55.5 years and an age range of 45 to 74 years3. Prevalence rates of clinical AD in DS vary substantially depending on the population studied’ which ranges from 4% in a community sample4 to 88% in an institution-based sample5. Many of the studies suffered from small sample sizes especially in the numbers of elderly DS persons included. A population-based study found that prevalence rates of AD increased with age – 8.9% (age 45-49), 17.7% (age 50-54), 32.1% (age 55-59) – up to age 60 years then decreased again – 25.6% (age above 60) – with an overall prevalence of 16.8%. The decrease was possibly accounted for by the increased mortality among the elderly demented DS persons6.
While congenital malformations and especially congenital heart defects are the commonest cause of mortality in early life, survival rates for people with DS have improved significantly over the years with advances in medical care and more are now living longer and into old age. Life expectancy was 9 years in 1929 and 12 in 1949 but more recent data indicate that the median age at death has increased from 25 years in 1983 to 49 years in 1997, an average increase of 1.7 years per year studied7. Survival beyond 60 years of age has also improved, with 44.4% and 13.6% of live born DS individuals now surviving to 60 and 68 years, respectively8. Recent evidence suggests that age, presence of dementia and mobility restrictions predict mortality in DS. When compared with the general population, it was found that impaired mobility, severity of learning disability, the presence of epilepsy and visual impairment, but not cardiovascular risk factors or sex, predicted survival9. There is mounting evidence that the presence of the APOEe2 allele increases longevity10 while non-demented DS individuals with at least one APOEe4 allele have increased mortality and up to five times the risks of dying within a 5- to 7-year follow-up period than those without an APOEe4 allele11.
The neuropathological manifestation of AD in DS has been attributed to the triplication and over-expression of the APP gene located on chromosome 2112. This neuropathology is invariably found in the brains of individuals with DS over the age of 40 years13. The characteristic neuropathological changes in AD include the deposition of beta-amyloid (Aß) as both extracellular diffuse and neuritic plaques as well as in meningeal and cerebral blood vessels and the intracellular accumulation of neurofibrillary tangles (NFTs) consisting of hyperphosphorylated tau in the form of paired helical filaments1. The accumulation of the diffuse plaques, which contain non-fibrillar amyloid, are commonly seen in DS before age 50 years and are not associated with AD whereas the neuritic plaques, which contain fibrillized amyloid and accumulate most commonly after age 50 years in DS, are associated with neuronal degeneration and loss of function10. NFTs occur at a later age in DS brains than amyloid plaques14 and have been associated with dementia status in DS3.
Aß, which consists of the two major species Aß 1-40 and Aß1-42, is produced from ß-amyloid precursor protein (APP) by sequential proteolytic cleavage by ß- and γ-secretases15. Starting from a young age, Aß 1-42 precedes Aß 1-40 in the course of amyloid deposition in the brains of people with DS16. Plasma Aß levels are also elevated in DS persons from childhood17. Plasma levels of Aß 1-40 and Aß 1-42 may be correlated with age in older DS adults18 and plasma Aß 1-42 levels were found to be higher in DS persons with dementia compared to those without in some studies19 but not others, which found no association between plasma Aß 1-40 and Aß 1-42 levels and the presence of dementia, APOE genotype or duration of dementia20 or age of onset21.
Genetic Factors
Studies in the general population with AD have identified mutations in APP, presenilin 1 (PS-1) and presenilin 2 (PS-2) to be associated with early-onset familial forms of AD and the APOE gene polymorphisms with the more common late-onset AD. However, in DS persons, PS-1 polymorphisms do not appear to have the same detrimental effects and are not associated with the development of dementia22.
Apolipoprotein E, which is involved in cholesterol transport and lipid metabolism and occurs in three allelic forms as e2, e3 and e4 on chromosome 19, has been shown to modulate the risk of AD in the general population23 with the APOEe4 allele associated with both late-onset familial and sporadic AD and the APOEe2 allele conferring a protective effect on the risk of developing AD24. In persons with DS, the relationship between APOEe4 and risk of AD remains controversial. Some studies have shown APOEe4 allele to be associated with increased risk of AD in persons with DS25-26 while others have not27,28. A recent meta-analysis showed APOEe4 to be significantly associated with the prevalence of AD in DS29.
The extended tau haplotype occurs in two forms, as H1 and H2, and has been associated with earlier onset of AD in the presence of the APOEe4 allele in the general population. Individuals with DS are at increased risk of developing dementia before age 45 years if they are heterozygous for the extended tau haplotype. Its lack of association with the APOE genotype suggests that it may be an independent risk factor for early onset dementia in DS30.
The genes for proteins that regulate tau phosphorylation, DYRK1A (dual-specificity tyrosine-regulated kinase 1A) and calcipressin, are located on chromosome 21 and their increased expression in DS may promote NFT formation. Furthermore, increased expression of both occur in response to increased Aß load and helps explain why NFTs occur at a later stage in the dementia process31.
Tetranucleotide repeat in intron 7 of APP (attt5-8) has been associated with earlier age of onset of dementia by up to 13 years in DS21.
GENDER AND THE ROLE OF OESTROGENS
Women in the general population are at increased risk of developing AD compared with men and oestrogen deficiency has been implicated in the cascade of pathological processes leading to AD. This has been reviewed recently32.
The evidence for the effect of gender on risk of AD in DS is contradictory, with some studies finding no differences in age of onset33, earlier onset34 or later onset35. Postmenopausal DS women with low levels of bioavailable oestradiol have been found to have an increased risk of developing AD36. The average age of menopause in DS women was younger than in the general population and the age of onset of AD correlated with the age of menopause, hence it has been suggested that oestrogen deficiency may be a possible independent risk factor for AD in DS37.
BRAIN METABOLITES: THE POTENTIAL ROLE OF MYO-INOSITOL
The Na+/myo-inositol co-transporter gene (SCL5A3) is located on chromosome 21 and myo -inositol significantly affects neuronal development and survival, neuronal osmolarity, membrane metabolism, signal transduction, protein kinase C activation and amyloid deposition38.
Proton magnetic resonance spectroscopy studies in healthy DS persons have reported significant increases in brain choline and myo- inositol, but no differences in brain NAA or creatine, and it has been suggested that the abnormal membrane turnover/degradation and increase in myo -inositol reflect a ‘predementia’ phase in which the neuropathology of AD is accumulating and precedes the loss of neurons39,40. The increased brain myo-inositol concentrations in healthy DS persons have been shown to be negatively correlated with overall cognitive and memory abilities41.
In a study comparing brain metabolites in demented, mild cognitively impaired (MCI) and healthy controls in the general population
Figure 48.1 The percentage change in unadjusted averages of hippocampal concentrations of NAA and mI by group comparisons (i.e. DS+ to DS-, AD to HC, DS- to HC and MCI to HC)
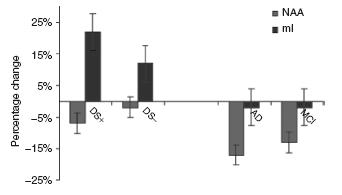
with demented (DS+) and non-demented (DS-) DS individuals, NAA concentrations were found to be reduced in persons with AD and MCI in the general population but not in either the demented or non-demented DS persons, suggesting that dementia in DS is associated with comparatively small reductions in viable neuronal function (as measured by NAA). In contrast, it was found that brain myo -inositol (mI) concentration was only increased in the demented DS group (see Figure 48.1), suggesting that elevated brain myo – inositol modifies the risk of dementia in people with DS possibly by interacting synergistically with other risk factors for dementia in this vulnerable population42. Research is currently under way to determine if manipulation of brain myo-inositol in DS by the use of lithium could offer a novel target for intervention in the dementia process in DS.
Free radicals produced by metabolic processes are kept in check by antioxidant defences and if this balance is disturbed, results in the highly reactive free radicals reacting with lipids in cell membranes to cause cellular damage, which contributes to neurodegeneration. The genetic locus for Cu,Zn-superoxide dismutase (SOD1), a key enzyme in free radical metabolism, is located on chromosome 2143 and has been suggested to have particular relevance to neurodegeneration in DS44.
There is increasing evidence to suggest that cytokines have an important role to play in the development and/or progression of AD in the general population. The inflammatory component of AD is dominated by the presence of activated microglial cells45. Studies have shown some support for the hypothesis that cytokines may act as a risk factor for the development of dementia in DS46,47. A small study found positive correlations between serum IL-6 levels with age and severity of AD in people with DS48, and there is preliminary evidence to indicate that serum TNF-a levels are elevated in DS persons with AD49, suggesting a disease-stage-dependent general activation of the immune system.
Using magnetic resonance imaging (MRI) based measurements of grey matter atrophy as a surrogate marker of neuronal density, various neuroimaging studies have described the macroscopic neuroanatomy of DS. More recent studies using sensitive quantitative measures of region-specific atrophy based on high-resolution MRI have suggested that age-specific atrophy in DS resembles the pattern of brain atrophy in the early stages of AD50.
In DS persons, overall brain volume including the cerebellum and cerebral grey and white matter, ventral pons, mammillary bodies and hippocampus is reduced whereas the parahippocampal gyrus are enlarged51. Reductions in cerebral volume are prominent in the frontal and occipital lobes and there is relative preservation of parietal lobe grey and temporal lobe white matter52.
As they age, non-demented DS individuals show volumetric reductions in most cortical brain regions53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






