Effects of Hormones on Seizure Expression
Torbjörn Bäckström
Introduction
Epilepsy is a complicated condition that may affect different areas of life. For example, fertility, family planning, and pregnancy are affected by epilepsy in part because of epilepsy-related alterations in hormones. Seizures and epilepsy also have effects on thyroid and adrenal hormones. Thyroid, adrenal, and sex steroid hormones affect the central nervous system (CNS), ultimately altering seizures and epilepsy. This chapter examines how endogenous and exogenous steroid hormones affect the CNS. Metabolites of the gonadal steroid progesterone and the adrenal steroid desoxycorticosterone seem to be of great importance for the hormonal effects on CNS excitability. They have all a similar chemical structure in being 3α-hydroxy-5α-pregnane steroids. The nomenclature of these substances is somewhat confusing because the same steroid can have several names. The progesterone metabolite 3α-hydroxy-5α-pregnane-20-one is also called allopregnanolone; in this chapter we use the abbreviation 3α5α-P. The 3α-hydroxy-5α-desoxycorticosterone is also called tetrahydrodesoxycorticosterone, for which THDOC is often used as an abbreviation.
Adrenocorticotropic Hormone Effect on Seizure Expression
Adrenocorticotropic Hormone
An anticonvulsant effect of adrenocorticotropic hormone (ACTH) in epileptic patients was first described by Klein and Livingston.87 Subsequently, ACTH was shown to be an effective treatment for patients with infantile spasms,144,146 cryptogenic epilepsy, and symptomatic seizures following organic brain lesions.118 Natural ACTH has fewer side effects than glucocorticoid hormones144 and does not induce seizures.86 Long-term glucocorticoid treatment, however, inhibits the activity of hypothalamic–pituitary–adrenal axis, which is a serious side effect. ACTH levels may be low in adult patients with epilepsy treated with antiepileptic drugs (AEDs), suggesting dysfunction of the adrenocortical axis.49,118,122,132,145 In 27 men and women with epilepsy, the mean serum ACTH level was lower than that in a control group, and this reduction was most pronounced in those with more severe epilepsy of longer duration.122 An ACTH level was evaluated in ten children before and after 6 months of therapy with valproic acid. Before treatment, ACTH values were normal, but they decreased significantly with treatment.78 Seizures may also alter ACTH release. A significant increase in ACTH occurred in ten patients within 60 minutes of a generalized tonic–clonic seizure.53 This elevation of ACTH suggests that the hypothalamic–pituitary–adrenal cortex axis may be affected by generalized seizures.
Corticosteroids
Adrenal cortical hormones exert regulatory effects on central nervous system (CNS) excitability.174 There is a significant increase in serum cortisol level in patients after generalized tonic–clonic seizures. In the rat’s brain, concentrations of neuroactive steroids increase with stress.140 Changes in cortisol levels may respond to physiologic stress associated with seizures.1 In a group of 45 men with epilepsy not treated with AEDs, cortisol levels were lower than in a control group.132 After AED treatment, levels decreased further, suggesting that AEDs influence adrenal cortex function.132 Further studies should evaluate the way in which alterations in the hypothalamic–pituitary–adrenal cortex axis are related to epilepsy or AEDs.
A relationship between adrenocortical steroids and seizures was found in several experimental and some clinical studies.66,141 Both mineralocorticoids and glucocorticoids alter neural activity,80 and the adrenal produces both pro- and anticonvulsant steroids125 (see later discussion). Aird and Gordon2 as early as 1951 showed that desoxycorticosterone has an anticonvulsant effect in patients with epilepsy. THDOC, a metabolite of desoxycorticosterone, is a potent γ-aminobutyric acid A (GABAA)–receptor agonist with nearly the same inhibitory effects as 3α5α-P (see later discussion).90,134,160 Although cortisol on its own increases brain excitability and decreases chloride flux through the GABAA receptor, when it is used together with 3α5α-P and THDOC the effect changes, and cortisol enhances the GABA agonistic effect of 3α5α-P and increases neural inhibition.160,169 The classic hormonal mineralocortico- and glucocorticoid receptors are present in the brain, and their expression has effects on neural activity in the regions in which they are located, as in the hippocampus.80 Several mechanisms are therefore likely to operate in parallel, but one can assume that rapid effects within minutes are mediated via the membrane-bound receptors whereas slower effects are via the classical hormonal receptors. More information is also needed regarding the direct role of adrenocortical hormones in seizure expression in patients with epilepsy.
Sex Hormones
Experimental Data on Excitability
Estradiol decreases the electroshock seizure threshold in rats in a dose-dependent mode.176 In humans, estrogen activates epileptiform electroencephalogram (EEG) discharges. Increased spike frequency occurred in 11 of 14 women with partial epilepsy given an intravenous (IV) injection of 40 mg of the estrogen Premarin. Generalized tonic–clonic seizures were
provoked within 15 minutes in 4 of the patients.102 An epileptic focus with 3/s spike-and-wave activity can be induced by applying estrogen directly on the cerebral cortex.111 The convulsive estrogen effect is not consistent, however, and pretreatment with estrogen was neuroprotective against N-methyl-D-aspartate (NMDA)–induced seizures in female ovariectomized rats and did not affect any of the seizure parameters measured in the male rats.82
provoked within 15 minutes in 4 of the patients.102 An epileptic focus with 3/s spike-and-wave activity can be induced by applying estrogen directly on the cerebral cortex.111 The convulsive estrogen effect is not consistent, however, and pretreatment with estrogen was neuroprotective against N-methyl-D-aspartate (NMDA)–induced seizures in female ovariectomized rats and did not affect any of the seizure parameters measured in the male rats.82
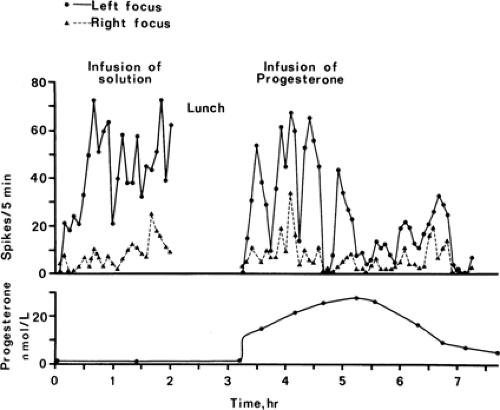
In contrast, progesterone in large doses induces anesthesia in humans116 and animals,58 increases the electroshock seizure threshold in animals, and protects against metrazol-induced seizures.158 In oophorectomized cats, progesterone at plasma levels equivalent to those of pregnancy significantly decreased the frequency of interictal spikes from penicillin foci.95 Progesterone at plasma levels similar to the luteal phase of the menstrual cycle significantly reduced interictal spike frequency in four of seven women with partial epilepsy. The women with low plasma protein binding progesterone showed the best response with the progesterone infusion, whereas women with the highest plasma protein binding did not respond.15
Progesterone metabolites that are reduced in the 5 position of the steroid molecule are very potent in their central nervous system (CNS)–depressant action, particularly those steroids with a 3α-hydroxy-5-reduced molecule.47 The antiepileptic effects of progesterone are mediated by these metabolites.119,142 In a rat model 3α5α-P is about eight times more potent, on a weight basis, in its anesthetic action than the most potent barbiturate known, methohexital.129 The 5α derivative of the preceding steroid seems to be somewhat more potent than the 5β derivative.129 In oophorectomized cats with a penicillin-induced epileptic focus, 3α5α-P is more potent in inhibiting epileptic activity than clonazepam.94 5β-Pregnenolone also acts as an anesthetic.35
In conclusion, estradiol increases brain excitability, whereas progesterone and some of its 5-reduced metabolites have potent inhibitory effects (Fig. 1). Wooley and Timiras175,176 showed an estrus cycle–dependent variation in electroshock seizure threshold, and other studies have shown an estrus cycle variation and male versus female difference in pentylenetetrazol seizure threshold.88 Interestingly, cycle-related changes and gender differences are described only for experimental models of epilepsy that use convulsant drugs specific for the GABAA receptor complex (GRC).75 In rats, sensitivity at the GRC also varies over the estrus cycle.48
Mechanisms Within the Brain
The classic target organ mechanism of steroid hormone action is via binding to an intracellular cytosol receptor, activating the genome for transcription and protein synthesis. Genome effects take at least 5 to 15 minutes, with some effects requiring hours to days.137 Steroid hormone effects on sexual behavior are probably medicated via this nuclear mechanism of action.137 Other possible CNS mechanisms of action of steroid hormones and their metabolites are by direct effects on neural membrane-bound receptors. Membrane effects are very rapid, occurring within a fraction of a second.7,137
The rapid antiepileptic effect is demonstrated with injections of 3α5α-P into the carotid artery of oophorectomized cats with an epileptic focus; epileptic spikes were instantaneously inhibited when the steroid solution reached the brain.94 These steroid effects are too rapid to be mediated via genomic activation and protein synthesis and represent direct effects on neural membrane-bound receptors. In electrophysiologic studies, responses to the excitatory amino acids quisqualate and NMDA were augmented by estradiol.89,155 Chronic treatment with estradiol may also sensitize the quisqualate system.155
3α5α-P acts as a receptor agonist at the GABAA receptor complex in the brain,52,109,110 similar to the action of benzodiazepines and barbiturates and some of the effects of alcohol. In mice, 3α5α-P inhibits convulsions induced by a number of convulsive substances acting at that GRC such as metrazol, (+)bicuculline, and picrotoxin but has no effect against maximal electroshock- and strychnine-induced seizures.21 These rapid actions of 3α5α-P are mediated via actions on the GRC-chloride ionophore complex.52 Progesterone metabolite effects on the GRC might mediate changes in seizure frequency over the luteal phase of the menstrual cycle. Metabolites of the
adrenocortical steroid THDOC have similar effects on the GABAA receptor.90
adrenocortical steroid THDOC have similar effects on the GABAA receptor.90
Accumulating evidence suggests that the neuroactive steroids are very similar to benzodiazepines also in their acute side effects and long-term actions. 3α5α-P induces sedation in humans and inhibition of learning when given IV to rats in the Morris water maze.81,161 Acute tolerance develops already after 90 minutes of 3α5α-P anesthesia.179 Increased seizure frequency and decreased seizure threshold occur at 3α5α-P withdrawal.143 At the molecular level, sustained high levels of neuroactive steroids reduce GABAA receptor responsiveness.38,177 3α5α-P effects in hippocampus vary between CA1 and dentate gyrus and change between different phases of the rat estrus cycle, indicating that a large diversity of effects and mechanisms can be expected.96
Another possible CNS mechanism of action of steroid hormones is by direct effects on monoamine turnover, metabolism, and receptors. Monoamines contribute to affective status and sexual behavior.45 Monoamine oxidase (MAO) and catechol-o-methyl transferase (COMT) in the rat brain are decreased by estradiol and increased by progesterone.32,75 In regularly menstruating women, platelet MAO, serotonin binding, and transport activity change during the cycle.22,173 Platelet MAO is considered the best peripheral reflection of brain MAO activity. In certain discrete regions of the hypothalamus of the diestrus rat, noradrenaline and dopamine turnover correlate with plasma estradiol and progesterone levels.100,101 Serotonin receptor expression changes in several brain areas during estrogen and estrogen plus progesterone treatments.25 Estradiol given to oophorectomized rats increases the turnover rate of dopamine in the hypothalamus,50 whereas progesterone increases serotonin turnover in limbic structures.92 Estrogen also alters dopamine turnover in the striatum, increasing dopamine receptor sensitivity there.76 The consequences of these effects on neurotransmission in relation to epilepsy are difficult to interpret.
Peripheral Neurosteroid Production
Estradiol and progesterone changes during the menstrual cycle and pregnancy are well described. Estradiol concentration increases tenfold from menstruation to ovulation. Progesterone increases 20 times from the follicular to the luteal phase. During menstruation, steroid hormones are at their lowest level. Both in humans and rats, 5α-reduced progesterone metabolites such as 5α-pregnane-3,20-dione (5-DHP) and 3α5α-P are produced peripherally and fluctuate in plasma and ovarian tissue in parallel with progesterone. In humans, 5α-DHP and 3α5α-P increase during the luteal phase of the menstrual cycle.74,77,167 3α5α-P and 5α-DHP are produced by the corpus luteum.8,133,139 During pregnancy, 3α5α-P and 5α-DHP increase about ten times and achieve about one third of the plasma concentration of progesterone.99,105 The concentration in fetal blood is about ten times that in maternal blood.99 Enzymes necessary for 5-reduction of progesterone are in the CNS.84 Brain concentrations of 5α-DHP in progesterone-induced anesthesia are much higher than those in plasma, indicating that progesterone is metabolized to 5α-reduced steroids within the brain.27 During stress and diurnal variation 3α5α-P and THDOC are produced from adrenal cortex with a short delay after glucocorticoids in both animals and humans.20,43,140
In plasma, estradiol is largely bound to transport proteins, sex-hormone–binding globulin (SHBG), and albumin, and only a small fraction (1%–3%) is unbound.157 Progesterone is bound to transcortin and albumin and about 10% is unbound.10,171 The 5α-reduced progesterone metabolites bind to albumin with low affinity.171 The unbound fraction exerts the normal effects in peripheral organs.
Steroid Distribution and Metabolism in the Central Nervous System
Estradiol114,135,136 and progesterone5,28 accumulate in specific brain regions in both rats and rhesus monkeys. The classic hormone-specific intracellular receptors have been identified in these regions104,137 and fulfill all the criteria for classic steroid target organs.114 The distribution of the estrogen alpha-receptor is different from that of the estrogen beta-receptor.131 The estradiol concentrations vary with the phase of the menstrual cycle in humans and the estrus phase in female rats.28,29 In addition, the progesterone CNS concentrations vary with ovarian steroid production. In rats the progesterone concentration in cerebral cortex is about 300 times higher during times of high ovarian progesterone production than during times of low ovarian production, whereas in hypothalamus the increase is 8 times and in plasma it is 12 times.28 In humans the progesterone and 3α5α-P concentrations are also related to ovarian production and vary among CNS areas.26 There is a significant correlation between the plasma and cortex, striatum, and cerebellum concentrations of progesterone, but not between the hypothalamus and plasma concentrations.28,180 This raises the possibility that there are different mechanisms for progesterone accumulation depending on CNS area in the rat. In rhesus monkeys 60% of the luteal ovarian progesterone production is taken up by the brain.24
The synthesis and metabolism of progesterone in rat brain have been studied in vivo and in vitro. Progesterone is metabolized to 5α-DHP and further to 3α5α-P. This is the substance active on the GABAA receptor. The 3α5α-P is further 20α-reduced, and this diminishes the action on the GABAA receptor (for reviews see refs. 84 and 115). In the primate hypothalamus, progesterone is metabolized to 3α5α-P and 20α-hydroxypregnane-4-one-3-one.23 The enzyme 5α-reductase is required for the synthesis of the progesterone metabolite 3α5α-P, and it is found in several areas of the mouse brain152 and in the human fetal brain.153 Neurosteriodogenesis seems to be important in the developing brain, and disorders of the developing brain are related to disturbed neurosteroid synthesis. This is perhaps not surprising, given that neurosteroid concentrations in fetal blood are very high.55,72,99 Other steroids with GABAA-receptor antagonist properties, such as pregnenolone sulfate, are also produced in the brain.109
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree