Efficacy of Antiepileptic Drugs
W. Edwin Dodson
Martin J. Brodie
Introduction
Since 1962, when the Kefauver-Harris Drug Amendments were passed, proof of effectiveness and evidence of reasonable safety have been required to obtain regulatory approval from the Food and Drug Administration (FDA) before a drug can be marketed to the public in the United States. Previously, drugs could be marketed if they were safe and contained what the label indicated, but proof of efficacy was not required. Hence, the antiepileptic drugs that were available before 1962 came to market without scientific studies that established their efficacy in preventing seizures.
The efficacy of an antiepileptic drug is its ability to prevent or interrupt seizures. However, efficacy is only one of several components of a drug’s actions that contribute to its clinical usefulness or clinical effectiveness. Whereas the information about efficacy as an isolated variable can be determined readily in experimental animals, actual measures of efficacy generally cannot be obtained in humans because assessment of seizure-suppressing activity is confounded by the occurrence of adverse side effects. As a result, in patients, the term clinical efficacy or clinical effectiveness is more appropriate even though the term efficacy is widely used colloquially.46 This chapter addresses basic aspects of drug efficacy as well as the clinical effectiveness of antiepileptic drugs in clinical settings.
Basic Measures of Drug Efficacy
Efficacy has two key dimensions: (a) potency and (b) spectrum of activity against different types of seizures. Efficacy is quantified by the term drug potency, which refers to the amount of a drug that is needed to produce a defined action. The less of a drug required, the higher is the potency. In measuring antiepileptic action, drug potency can be expressed in several ways. One of the most common ways is to describe the dose of drug that prevents seizures in 50% of experiments, which is abbreviated as ED50. Potency could also be expressed in other ways, such as the minimally effective dose or as the concentration of medication that produces the desired action.
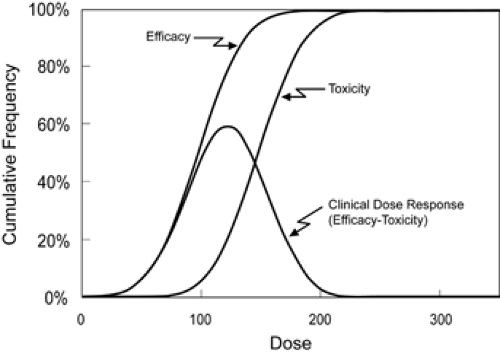
In animal models used for screening antiepileptic drugs, seizures are induced by various techniques such as electroshock or chemoconvulsant administration. These models provide standardized systems in which progressively increasing doses of a drug can be evaluated (Fig. 1). However, due to concerns about drug-induced adverse effects that might occur when very high doses are administered to humans, a comprehensive assessment of efficacy per se cannot be obtained in people. Instead, what is measured and what is most important in patients is clinical effectiveness.
Drug toxicity can be quantified in animals using approaches similar to those used to determine efficacy except that the experimental endpoint is a toxic outcome. The rotorod test is a widely used measure of toxicity for antiepileptic drugs in animals. Although this test has little relevance to humans, we mention it here because it has a well-defined outcome and illustrates an idealized measure of drug toxicity. The experimental animal, usually a mouse, is placed on top of a slowly rotating rod. Normal mice can maintain their balance on the rod indefinitely; intoxicated mice fall off the rotating rod, thereby providing a clear experimental endpoint of drug intoxication. By administering progressively larger doses to groups of mice and then testing their ability to stay atop the rotating rod, one can find the dose that produces toxicity in 50% of the mice (TD50). No such clear-cut test exists for evaluating antiepileptic drug toxicity in humans. Furthermore, adverse effects of medications in rodents generally provide little insight into the toxicities that occur in people.
Whereas side effects impose limits on the dose of a drug that can be given, to determine the usefulness of a drug, information is needed about both drug efficacy and drug toxicity. The parameter that combines these measurements is the therapeutic index (TI). The therapeutic index is the ratio of the dose that produces toxicity in half of animals to the dose that is effective in half (TI = TD50 ÷ ED50). The larger the therapeutic index, the wider is the margin of safety for the drug and the more likely it is that it will be clinically useful. The wider the therapeutic index, the higher is the percentage of people who are likely to benefit without limiting adverse effects. FIGURE 1 was constructed for a drug with a relatively narrow therapeutic index of 1.5 and results in a clinical dose–response curve on which the best-tolerated dose would benefit only 60% of patients.
Table 1. Factors that influence antiepileptic drug effectiveness | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The therapeutic index indicates the potential clinical effectiveness of a drug in patients. The limit of clinical usefulness of a drug is determined by its tolerability, where the term tolerability describes the collective toxicities of a drug that would lead to its being discontinued. In this chapter, the term clinical effectiveness is the clinical counterpart of the basic concept of the therapeutic index.
The critical contribution of toxicity to the clinical usefulness of a drug is illustrated by the drugs clonazepam and valproic acid. Among the available antiepileptic drugs, clonazepam is most potent, but it is among the least useful because of its toxicity profile and low tolerability. For practical purposes, clonazepam has an antiepileptic therapeutic index of approximately 1, which is to say that the dose that prevents seizures also causes adverse effects. On the other hand, compared to other antiepileptic drugs, valproic acid has low potency, but it is highly useful because of its even lower toxicity and better tolerability. This results in a larger therapeutic index than that of clonazepam and greater clinical usefulness. Thus, the relationship between efficacy and toxicity—the
therapeutic index—is a key determinant of a drug’s effectiveness and practical value. Whereas individual people differ in their sensitivity to developing side effects to a given drug, from the perspective of an individual person, the clinical effectiveness of the drug is related to the risk-to-benefit ratio for that individual.24,46,52
therapeutic index—is a key determinant of a drug’s effectiveness and practical value. Whereas individual people differ in their sensitivity to developing side effects to a given drug, from the perspective of an individual person, the clinical effectiveness of the drug is related to the risk-to-benefit ratio for that individual.24,46,52
Besides the efficacy and tolerability of an antiepileptic drug, several other factors help to determine the extent to which a drug is clinically useful (Table 1). These can be categorized as patient related, epilepsy related, and drug related. Epilepsy-related factors dictate the clinical conditions in which a drug might be used. Patient-related factors influence how a drug might be suited for use in various groups of patients. Drug-related factors, such as the pharmacokinetics of the drug, affect the drug’s ease of administration and have a major impact on a drug’s usefulness. Drug availability and cost put practical limits on drug usefulness as well.
Methods for Establishing Drug Effectiveness
Determining the effectiveness of an antiepileptic drug is a lengthy process that evolves over time. Preclinical studies that are done to satisfy regulatory requirements start with dose-ranging and other pilot studies that provide insight into how to design scientifically sound clinical trials that can document beneficial drug action. A minimum of two clinical trials must be done, but often several more are completed before a drug is approved for public use.
Drug approval requires randomized, controlled trials. These are conducted in groups of volunteer patients with defined clinical features that are selected to minimize subject-to-subject variation and to conform to ethical human studies guidelines. Experimental controls are essential in new drug evaluation because in even the most severe forms of epilepsy, seizure frequencies fluctuate over time, resulting in appreciable placebo effects. For example, cinromide, a drug investigated in the 1970s and 1980s, appeared promising in pilot studies in preventing frequent seizures associated with the Lennox-Gastaut syndrome.65 A randomized trial, however, disclosed that cinromide’s effect was no different than placebo, and hence it was shown to be ineffective.16
A wide array of research designs has been used to establish antiepileptic effectiveness in humans (Table 2). Different
research designs require different endpoints depending on considerations such as the duration of epilepsy among patients (chronic vs. new onset), seizure frequency, seizure severity, and characteristics of the group selected for study. For both practical and ethical reasons, during the initial phases of new drug evaluation, studies are conducted in patients with severe epilepsy whose seizures have not been controlled with available medications.
research designs require different endpoints depending on considerations such as the duration of epilepsy among patients (chronic vs. new onset), seizure frequency, seizure severity, and characteristics of the group selected for study. For both practical and ethical reasons, during the initial phases of new drug evaluation, studies are conducted in patients with severe epilepsy whose seizures have not been controlled with available medications.
|
Study Designs and Experimental Endpoints
Drug testing starts in patients who have epilepsy with uncontrolled seizures. Whereas these patients have the most to gain by participation in drug trials, the refractory nature of their seizures to medication makes them a challenging group in whom to show effectiveness of a new drug. The most common research design is adjunctive (also called add-on) parallel-group design in which a group of patients with uncontrolled epilepsy are randomly assigned to treatment with the investigational agent or with an inactive placebo. Later in the drug development process, the drug may be evaluated as monotherapy depending on the promise of the drug in terms of its clinical effectiveness and the manufacturer’s priorities and resources. As discussed later, despite extensive preclinical testing, information about how to use new drugs in the population at large is patchy and incomplete at the time when drugs are first approved for marketing.22
Traditional measures of antiepileptic drug effectiveness in adjunctive studies include percentage seizure reduction from the baseline period, percentage of patients rendered seizure free, and responder rate. Change in seizure frequency is the most widely used outcome measure and is usually expressed as mean or median percentage reduction. This measure compares the seizure frequency during the treatment period to the seizure frequency during the baseline period before the investigational drug is administered. In add-on trials of new drugs, so few patients become seizure free that cessation of seizures is rarely a useful outcome measure.15
The responder rate is a widely used outcome when change in seizure frequency is the primary outcome measure.1,3,45 This is the proportion of subjects whose seizure frequency decreases by various percentages from the baseline period. The proportion that is most often reported is the 50% responder rate. The 50% responder rate is the percentage of patients who experience a 50% or greater reduction in seizure frequency while taking the investigational compound as compared to their baseline seizure frequency. Other levels of response such as the 75% responder rate can be used as well,8,19,35,48 but the 50% rate appears most often because higher responder rates are uncommon in new antiepileptic drug trials. Representative 50% responder rates during clinical trials of newer antiepileptic drugs introduced since 1992 are in the range of 20% to 50%.
Seizure severity outcomes depend on the use of rating scales or seizure scores. A number of scales have been developed to evaluate seizure severity, adverse drug effects, and overall quality of life, but there is no consensus regarding which scales are most useful. Overall, however, the cessation of seizures has the greatest beneficial impact as measured by quality-of-life scales.13 On most scales, frequent and generalized convulsive seizures are scored as being more severe than infrequent seizures and nonconvulsive partial or absence seizures.7,14,46,63 Note that scales for quantifying adverse effects can be better predictors of a person’s completing a study than seizure counts. For example, quality-of-life measurements using the Side Effect and Life Satisfaction (SEALS) Inventory correlated better with study completion than seizure counts in a double-blind comparison of lamotrigine and carbamazepine.29
The response ratio has been used infrequently as an investigational endpoint, mainly for gabapentin trials. It is the difference in seizure frequencies during the treatment period minus the frequency during baseline, which is then divided by the sum of the seizure frequencies during both periods.26,27 The response ratio has no dimensions, and results expressed in this way are intuitively difficult to use.
The outcome of time to the nth seizure has also been used in presurgical monotherapy trials.21,46,51,53,54 This endpoint is versatile, and it offers a measure of protection for patients by limiting the number of seizures that they need to experience before exiting the study and moving on to another treatment. Whereas the time to nth seizure outcome is useful in establishing drug effectiveness, it has not been used widely because allowing patients to have seizures while receiving only placebo or a suboptimal therapy, albeit under watchful supervision, introduces risk and raises ethical concerns.
Besides ethical concerns, there are methodologic pitfalls to the time-to-nth-seizure endpoint. Whereas time to nth seizure can rapidly detect antiepileptic effects in patients with high seizure frequencies, it is not useful for comparing the effectiveness of different drugs. Moreover, applying this endpoint can also be tricky, and if it is not used properly, a beneficial drug action can be missed. For example, if the nth-seizure criterion is set at, say, the second or third seizure and if the investigational drug is more effective than expected, the number of seizures that occurs in the study may be insufficient to permit the detection of a difference between treatment groups. This outcome occurred with a monotherapy trial of topiramate in which the time of the second seizure was chosen as the primary outcome variable but the patients had insufficient numbers of seizures during the study.31 When the study was repeated with the endpoint set to time of the first seizure, the effectiveness of the drug was documented.4
Monotherapy Trials
Some drugs that show promise in polytherapy or adjunctive trials are subsequently tested in monotherapy. Whereas add-on study designs actually compare various drug combinations, they do not scientifically establish the effectiveness of drugs used alone in monotherapy. Establishing a drug’s effectiveness in monotherapy requires randomized, controlled trials of the drug given alone. Monotherapy can be approached by eliminating other drugs from multidrug regimens or can be evaluated de novo in patients who are untreated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree